Thermosensitive Hydrogel-Functionalized Mesoporous Silica Nanoparticles for Parenteral Application of Chemotherapeutics
Abstract
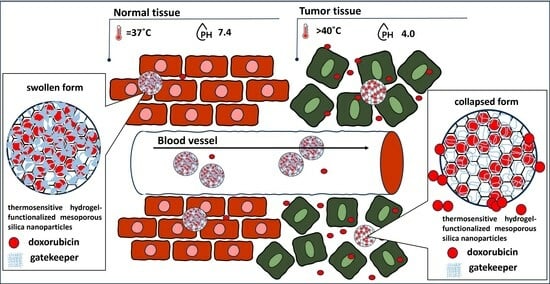
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Hydrogel-Functionalized Nanoparticles MCM-41/AA-g-PnVCL
2.2. Characterization of the AA-g-PnVCL and MCM-41/AA-g-PnVCL
2.2.1. FT-IR
2.2.2. TEM and SEM Analysis
2.2.3. TGA
2.2.4. XRD
2.2.5. Temperature-Sensitive Behaviour
2.2.6. Dynamic Light Scattering (DLS)
2.2.7. Dox Loading Efficiency
2.2.8. Drug Release
2.2.9. Haemolysis Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogel-Functionalized MCM-41 Nanoparticles
4.2.1. Synthesis of COOH-Modified MCM-41 Particles (MCM-41-COOH)
4.2.2. Synthesis of MCM-41-COOH/AA-g-PnVCL Nanoparticles
4.3. Characterization of the Hydrogel, the MCM-41 Nanoparticles and the Hydrogel-Functionalized Nanoparticles (MCM-41/AA-g-PnVCL)
4.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
4.3.2. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
4.3.3. Thermogravimetric Analysis (TGA)
4.3.4. X-ray Powder Diffraction (XRD)
4.3.5. Determination of the Lower Critical Solution Temperature (LCST)
4.3.6. Dynamic Light Scattering (DLS)
4.3.7. Drug Loading and Loading Efficiency
4.3.8. In Vitro Drug Release Study
4.3.9. Haemolysis Assay
4.3.10. Statistical analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Wang, X.-L.; He, D.-H.; Cheng, Y.-X. Protection against Chemotherapy- and Radiotherapy-Induced Side Effects: A Review Based on the Mechanisms and Therapeutic Opportunities of Phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef]
- Niu, G.; Cogburn, B.; Hughes, J. Preparation and Characterization of Doxorubicin Liposomes. In Cancer Nanotechnology: Methods and Protocols; Methods in Molecular Biology; Grobmyer, S.R., Moudgil, B.M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 211–219. ISBN 978-1-60761-609-2. [Google Scholar]
- Mal, A.; Prabhuraj, R.S.; Malhotra, R.; Valvi, S.K.; Ingle, A.; Srivastava, R.; De, A.; Bandyopadhyaya, R. pH-Responsive Sustained Delivery of Doxorubicin Using Aminated and PEGylated Mesoporous Silica Nanoparticles Leads to Enhanced Antitumor Efficacy in Pre-Clinical Orthotopic Breast Cancer Model. J. Drug Deliv. Sci. Technol. 2022, 77, 103800. [Google Scholar] [CrossRef]
- Moraes, S.; Marinho, A.; Lima, S.; Granja, A.; Araújo, J.P.; Reis, S.; Sousa, C.T.; Nunes, C. Targeted Nanostructured Lipid Carriers for Doxorubicin Oral Delivery. Int. J. Pharm. 2021, 592, 120029. [Google Scholar] [CrossRef]
- Valencia-Lazcano, A.A.; Hassan, D.; Pourmadadi, M.; Shamsabadipour, A.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. 5-Fluorouracil Nano-Delivery Systems as a Cutting-Edge for Cancer Therapy. Eur. J. Med. Chem. 2023, 246, 114995. [Google Scholar] [CrossRef]
- Shao, D.; Gao, Q.; Sheng, Y.; Li, S.; Kong, Y. Construction of a Dual-Responsive Dual-Drug Delivery Platform Based on the Hybrids of Mesoporous Silica, Sodium Hyaluronate, Chitosan and Oxidized Sodium Carboxymethyl Cellulose. Int. J. Biol. Macromol. 2022, 202, 37–45. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent Progress in Nanomedicine for Enhanced Cancer Chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537. [Google Scholar] [CrossRef]
- Slavkova, M.; Tzankov, B.; Popova, T.; Voycheva, C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels 2023, 9, 352. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment Sensitive Hydrogels for Drug Delivery Applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Chang, L.; Liu, S.; Gao, T.; Sang, X.; Zhang, Z.; Mu, W.; Liu, X.; Liang, S.; Yang, H.; et al. Temperature Sensitive Liposome Based Cancer Nanomedicine Enables Tumour Lymph Node Immune Microenvironment Remodelling. Nat. Commun. 2023, 14, 2248. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent Advances in Thermo-Sensitive Hydrogels for Drug Delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Thananukul, K.; Kaewsaneha, C.; Opaprakasit, P.; Lebaz, N.; Errachid, A.; Elaissari, A. Smart Gating Porous Particles as New Carriers for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 174, 425–446. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of Hydrogels: Linking the Nano to the Microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Waterhouse, G.I.N.; Zhang, Q.; Li, L. Poly(N-Isopropylacrylamide)/Mesoporous Silica Thermosensitive Composite Hydrogels for Drug Loading and Release. J. Appl. Polym. Sci. 2020, 137, 48391. [Google Scholar] [CrossRef]
- Rout, S.R.; Gowtham, K.; Sheikh, A.; Parvez, S.; Dandela, R.; Kesharwani, P. Chapter 15—Recent Advances and Future Prospective of Hybrid Drug Delivery Systems. In Hybrid Nanomaterials for Drug Delivery; Woodhead Publishing Series in Biomaterials; Kesharwani, P., Jain, N.K., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 357–374. ISBN 978-0-323-85754-3. [Google Scholar]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-Chitosan Oligosaccharide-ZnO Composite Hydrogel for Accelerating Wound Healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, Fabrication and Drug Release Potential of Dual Stimuli-Responsive Composite Hydrogel Nanoparticle Interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite Hydrogels with Embedded Silver Nanoparticles and Ibuprofen as Wound Dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, Y.; Lin, C.; Tao, L. Thermosensitive Hydrogel-Functionalized Gold Nanorod/Mesoporous MnO2 Nanoparticles for Tumor Cell-Triggered Drug Delivery. Mater. Sci. Eng. C 2021, 131, 112504. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Chen, G.-B.; Chen, H.-H.; Zhang, J.-B.; Li, H.-Z.; Sheng, M.-X.; Weng, W.-B.; Guo, S.-M. Cancer Cell Membrane-Cloaked Mesoporous Silica Nanoparticles with a pH-Sensitive Gatekeeper for Cancer Treatment. Colloids Surf. B Biointerfaces 2019, 175, 477–486. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Hu, X.; Hao, X.; Wu, Y.; Zhang, J.; Zhang, X.; Wang, P.C.; Zou, G.; Liang, X.-J. Multifunctional Hybrid Silica Nanoparticles for Controlled Doxorubicin Loading and Release with Thermal and pH Dual Response. J. Mater. Chem. B 2013, 1, 1109–1118. [Google Scholar] [CrossRef]
- Voycheva, C.; Popova, T.M.; Tzankova, V.; Stefanova, D.; Tzankova, D.; Spassova, I.; Kovacheva, D.; Tzankov, B. Doxorubicin and Quercetin Double Loading in Modified MCM-41 Lowered Cardiotoxicity in H9c2 Cardioblast Cells In Vitro. Bioengineering 2023, 10, 637. [Google Scholar] [CrossRef]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural Biopolymers as Smart Coating Materials of Mesoporous Silica Nanoparticles for Drug Delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Algal Phycocolloids: Bioactivities and Pharmaceutical Applications. Mar. Drugs 2023, 21, 384. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Factors Affecting Yield and Gelling Properties of Agar. J. Appl. Phycol. 2017, 29, 1527–1540. [Google Scholar] [CrossRef]
- Voycheva, C.; Slavkova, M.; Popova, T.; Tzankova, D.; Tosheva, A.; Aluani, D.; Tzankova, V.; Ivanova, I.; Tzankov, S.; Spassova, I.; et al. Synthesis and Characterization of PnVCL Grafted Agar with Potential Temperature-Sensitive Delivery of Doxorubicin. J. Drug Deliv. Sci. Technol. 2022, 76, 103725. [Google Scholar] [CrossRef]
- Alam Khan, S.; Jawaid Akhtar, M. Structural Modification and Strategies for the Enhanced Doxorubicin Drug Delivery. Bioorganic Chem. 2022, 120, 105599. [Google Scholar] [CrossRef] [PubMed]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bei, H.P.; Piao, Y.; Wang, Y.; Yang, M.; Zhao, X. Polymer-Brush-Grafted Mesoporous Silica Nanoparticles for Triggered Drug Delivery. ChemPhysChem 2018, 19, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Ghalehkhondabi, V.; Fazlali, A.; Soleymani, M. Temperature and pH-Responsive PNIPAM@PAA Nanospheres with a Core-Shell Structure for Controlled Release of Doxorubicin in Breast Cancer Treatment. J. Pharm. Sci. 2023, 112, 1957–1966. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in Cancer: Reviewing the Combination of Hyperthermia and Triggered Chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Kawakita, M.; Ogura, K. High Surface-Area Silica with Controlled Pore Size Prepared from Nanocomposite of Silica and Citric Acid. J. Phys. Chem. B 2000, 104, 12184–12191. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Férey, G.; Vallet-Regí, M. Influence of Superficial Organic Modification of MCM-41 Matrices on Drug Delivery Rate. Solid State Sci. 2006, 8, 1243–1249. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Cheng, Y.-M.; Yen, S.-K. Doxorubicin–Chitosan–Hydroxyapatite Composite Coatings on Titanium Alloy for Localized Cancer Therapy. Mater. Sci. Eng. C 2019, 104, 109953. [Google Scholar] [CrossRef]
- Hu, S.H.; Fang, R.H.; Chen, Y.W.; Liao, B.J.; Chen, I.W.; Chen, S.-Y. Photoresponsive Protein-Graphene-Protein Hybrid Capsules with Dual Targeted Heat-Triggered Drug Delivery Approach for Enhanced Tumor Therapy. Adv. Funct. Mater. 2014, 24, 4144–4155. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-Vinylcaprolactam), a Comprehensive Review on a Thermoresponsive Polymer Becoming Popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biodegradable and Thermo-Sensitive Chitosan-g-Poly(N-Vinylcaprolactam) Nanoparticles as a 5-Fluorouracil Carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Banihashem, S.; Nezhati, M.N.; Panahia, H.A. Synthesis of Chitosan-Grafted-Poly(N-Vinylcaprolactam) Coated on the Thiolated Gold Nanoparticles Surface for Controlled Release of Cisplatin. Carbohydr. Polym. 2020, 227, 115333. [Google Scholar] [CrossRef] [PubMed]
- Karesoja, M.; McKee, J.; Karjalainen, E.; Hietala, S.; Bergman, L.; Linden, M.; Tenhu, H. Mesoporous Silica Particles Grafted with Poly(Ethyleneoxide-Block-N-Vinylcaprolactam). J. Polym. Sci. Part Polym. Chem. 2013, 51, 5012–5020. [Google Scholar] [CrossRef]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive Mesoporous Silica Nanoparticles for Triggered Drug Release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef]
- Galhano, J.; Marcelo, G.A.; Duarte, M.P.; Oliveira, E. Ofloxacin@Doxorubicin-Epirubicin Functionalized MCM-41 Mesoporous Silica-Based Nanocarriers as Synergistic Drug Delivery Tools for Cancer Related Bacterial Infections. Bioorg. Chem. 2022, 118, 105470. [Google Scholar] [CrossRef]
- Miao, Y.; Feng, Y.; Bai, J.; Liu, Z.; Zhao, X. Optimized Mesoporous Silica Nanoparticle-Based Drug Delivery System with Removable Manganese Oxide Gatekeeper for Controlled Delivery of Doxorubicin. J. Colloid Interface Sci. 2021, 592, 227–236. [Google Scholar] [CrossRef]
- Yan, J.; Xu, X.; Zhou, J.; Liu, C.; Zhang, L.; Wang, D.; Yang, F.; Zhang, H. Fabrication of a pH/Redox-Triggered Mesoporous Silica-Based Nanoparticle with Microfluidics for Anticancer Drugs Doxorubicin and Paclitaxel Codelivery. ACS Appl. Bio Mater. 2020, 3, 1216–1225. [Google Scholar] [CrossRef]
- Dasgupta, D.; Das, M.; Thakore, S.; Patel, A.; Kumar, S.; Seshadri, S. Development of a Controlled Sustainable Anticancer Drug Delivery Nanosystem Comprising Doxorubicin and Functionalized MCM-48. J. Drug Deliv. Sci. Technol. 2022, 72, 103419. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.-Y. Light- and pH-Responsive Release of Doxorubicin from a Mesoporous Silica-Based Nanocarrier. Chem. Weinh. Bergstr. Ger. 2011, 17, 3338–3342. [Google Scholar] [CrossRef]
- Sturgeon, R.J.; Schulman, S.G. Electronic Absorption Spectra and Protolytic Equilibria of Doxorubicin: Direct Spectrophotometric Determination of Microconstants. J. Pharm. Sci. 1977, 66, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.H.; Crommelin, D.J.A.; Storm, G.; Hulshoff, A. Doxorubicin Decomposition on Storage. Effect of pH, Type of Buffer and Liposome Encapsulation. Int. J. Pharm. 1985, 23, 1–11. [Google Scholar] [CrossRef]
- Hakeem, A.; Zahid, F.; Zhan, G.; Yi, P.; Yang, H.; Gan, L.; Yang, X. Polyaspartic Acid-Anchored Mesoporous Silica Nanoparticles for pH-Responsive Doxorubicin Release. Int. J. Nanomed. 2018, 13, 1029–1040. [Google Scholar] [CrossRef]
- Maghsoudi, M.; Abbasian, M.; Farhadi, K.; Mahmoodzadeh, F.; Ghorbani, M.; Hoseinzadeh, M. Mesoporous Si-MCM-41/Polymer as a pH-Responsive Drug Delivery System for Cancer Therapy. ChemistrySelect 2020, 5, 11901–11909. [Google Scholar] [CrossRef]
- Saroj, S.; Rajput, S.J. Tailor-Made pH-Sensitive Polyacrylic Acid Functionalized Mesoporous Silica Nanoparticles for Efficient and Controlled Delivery of Anti-Cancer Drug Etoposide. Drug Dev. Ind. Pharm. 2018, 44, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex Vivo Red Blood Cell Hemolysis Assay for the Evaluation of pH-Responsive Endosomolytic Agents for Cytosolic Delivery of Biomacromolecular Drugs. J. Vis. Exp. JoVE 2013, 73, e50166. [Google Scholar] [CrossRef]
- Available online: https://mu-sofia.bg/nauka/nauka/etika-nauchni-izsledvania/ (accessed on 14 September 2023).
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 14 June 2023).
- Romero, A.A.; Alba, M.D.; Zhou, W.; Klinowski, J. Synthesis and Characterization of the Mesoporous Silicate Molecular Sieve MCM-48. J. Phys. Chem. B 1997, 101, 5294–5300. [Google Scholar] [CrossRef]









| Parameter | MCM-41 | MCM-41/AA-g-PnVCL | MCM-41/AA-g-PnVCL/Dox |
|---|---|---|---|
| Size, nm | 217 ± 7.3 | 360 ± 8.4 | 369 ± 3.3 |
| PDI | 0.31 | 0.86 | 0.83 |
| Zeta potential, mV | −37.1 ± 3.7 | −26.6 ± 3.6 | −19.8 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voycheva, C.; Slavkova, M.; Popova, T.; Tzankova, D.; Stefanova, D.; Tzankova, V.; Ivanova, I.; Tzankov, S.; Spassova, I.; Kovacheva, D.; et al. Thermosensitive Hydrogel-Functionalized Mesoporous Silica Nanoparticles for Parenteral Application of Chemotherapeutics. Gels 2023, 9, 769. https://doi.org/10.3390/gels9090769
Voycheva C, Slavkova M, Popova T, Tzankova D, Stefanova D, Tzankova V, Ivanova I, Tzankov S, Spassova I, Kovacheva D, et al. Thermosensitive Hydrogel-Functionalized Mesoporous Silica Nanoparticles for Parenteral Application of Chemotherapeutics. Gels. 2023; 9(9):769. https://doi.org/10.3390/gels9090769
Chicago/Turabian StyleVoycheva, Christina, Marta Slavkova, Teodora Popova, Diana Tzankova, Denitsa Stefanova, Virginia Tzankova, Ivelina Ivanova, Stanislav Tzankov, Ivanka Spassova, Daniela Kovacheva, and et al. 2023. "Thermosensitive Hydrogel-Functionalized Mesoporous Silica Nanoparticles for Parenteral Application of Chemotherapeutics" Gels 9, no. 9: 769. https://doi.org/10.3390/gels9090769