Optimization of the Methods to Develop Stable Polymer Gels for Water Management in Medium- and Ultra-High-Salinity Reservoirs
Abstract
:1. Introduction
- (1)
- The use of temperature and salt-resistant polymers. Two approaches are usually employed here. The first involves using a hydrophobic association polymer (e.g., AP-P4, AP-P5, MKY, etc.) or cationic-type polymer. The former type maintains a high viscosity via the association that occurs between its hydrophobic groups under HTHS conditions (which subsequently improves the stability of its corresponding gels). Using associated polymers such as AP-P4, researchers have managed to develop temperature- and salt-resistant gels that are stable at 95 °C and 16.0 × 104 mg/L [6,7,8,9]. In contrast, cationic polymers are positively charged and therefore tend to exclude other cations in water (especially Ca2+/Mg2+ ions). This reduces the effect of those cations on the stability of the gel. Zhou and Xu, for example, used cationic polyacrylamide (CPAM) in place of conventional hydrolyzed polyacrylamide (HPAM) and thus developed a cationic polymer gel [10,11]. The gel was found to have good stability under the test conditions used: a temperature of 85 °C and an ultra-high salinity of 22.0 × 104 mg/L (Ca2+/Mg2+: 10,000 mg/L). The second approach is to use special copolymers and terpolymers. In particular, some special temperature- and salt-resistant monomers can be introduced. In this way, for example, 2-acrylamido-2-methylpropane sulfonic acid (AMPS), N-vinyl pyrrolidone (NVP), N-vinylacetamide (NVA), dimethylaminoethyl methacrylate (DMAM), etc., can be introduced into polymers such as polyacrylamide (PAM). This inhibits the hydrolysis of PAM at high temperatures, which reduces the formation of carboxylic acid groups in the PAM polymer and significantly enhances its temperature and salt resistance. Gailard, Zhu, and Ye found that PAMs containing NVP monomers can be rendered stable even at a temperature of 150 °C [12,13,14]. The polymers remained stable for five months and hence exhibited good temperature resistance. Copolymer gels based on acrylamide and AMPS have also been focused upon by many researchers, and a whole class of polymer gels that are stable up to a maximum temperature and salinity of 130 °C and 22 × 104 mg/L, respectively, have been developed [15,16,17,18,19,20,21,22]. Hsieh et al. reported that terpolymers made of AM/AMPS/NVP possessed excellent thermal stability and aged well in seawater at temperatures up to 149 °C [23]. Lu et al. prepared a polymer gel by copolymerizing acrylamide and acrylonitrile (AN) and found that it remained stable for 12 months at 105 °C [24]. The polyvinyl alcohol (PVA), phenol, and formaldehyde gel system prepared by Hoskin et al. was found to dehydrate less than 5% after aging for 70 days at 204.4 °C [25]. PVA gels have also been shown to be thermally stable by Victorius and Shu [26,27].
- (2)
- The use of temperature and salt-resistant crosslinking agents. Gels can be prepared by replacing the traditional metal ions or phenolic system with crosslinking agents such as polyethylenimine (PEI). In this way, people have prepared polymer gels that are stable at the highest evaluation temperature used of 177 °C [28,29,30,31,32,33]. Although these gels have good temperature and salt resistance, they have an obvious deficiency: a high concentration of PEI crosslinker needs to be used, which makes this approach uneconomical.
- (3)
- Adding a complexant to act as a stabilizer. Agents can be employed that form complexes with divalent ions and thus deactivate them. Such complexants thus resist the occurrence of gel syneresis that results from the over-crosslinking of sites featuring carboxyl functional groups caused by divalent ions. Albonico et al. systematically investigated the effect on the stability of polymers and their corresponding gels resulting from the use of more than ten complexants (including EDTA, oxalate, citrate, and NTA) in water at 120 °C and containing 1.0 × 104 mg/L of salt [34]. They found that the addition of such agents significantly enhances the stability of the gel. Jia et al. and Wei et al. prepared polymer gels that were stable in seawater at 130 °C that contained 3.5 × 104 mg/L of salt [35,36]. In their work, however, organophosphates and a polyate (called WZ) were used as stabilizers.
2. Results and Discussion
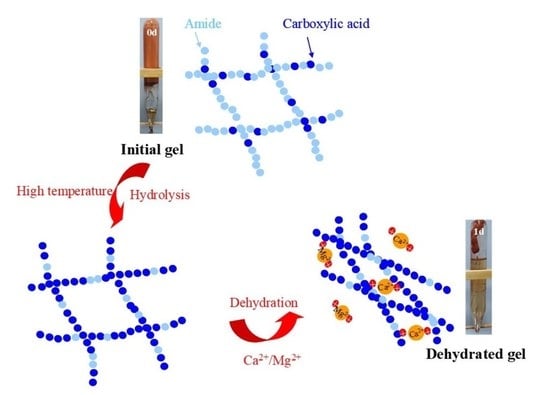
2.1. Syneresis Mechanism of Gel at HTHS
2.2. Gel Optimization for Medium-Salinity Conditions
2.2.1. Effect of Polymer Type and Concentration
2.2.2. Effect of Deoxidizer
2.2.3. Effect of Complexant as a Stabilizer
- (1)
- Gels stabilized by different complexants.
- (2)
- Ability of sodium oxalate to stabilize different polymer gels.
- (3)
- Salinity adaptability of complexant-stabilized gel.
2.3. Gel Optimization for Ultra-High-Salinity Conditions
2.3.1. Optimization of the Polymers (SAV10 and SAV55)
2.3.2. Plugging Capacity of Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Stability Tests
4.2.2. Core-Plugging Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, X.A.; Wang, U.F.; Wang, K.L. Fundamentals of Enhanced Oil Recovery; Petroleum Industry Press: Beijing, China, 2007. [Google Scholar]
- Bai, Y.; Liu, Y.; Yang, K.; Lang, Y. Application and research prospect of functional polymer gels in oil and gas drilling and development engineering. Gels 2023, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Bai, B.J.; Hou, J.R. Polymer gel systems for water management in high-temperature petroleum reservoirs: A chemical review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Randy, S.; Bergit, B. Water shutoff and conformance improvement: An introduction. Pet. Sci. 2021, 18, 450–478. [Google Scholar]
- Li, J.J.; Xiong, C.M.; Bai, Y.R.; Jiang, R.Y.; Wei, F.L.; Zhang, M. Leak-off behavior and water shut-off performance of a polymer/chromium (Cr3+) gel in fractured media. J. Cent. South Univ. 2017, 24, 1418–1429. [Google Scholar] [CrossRef]
- Yang, Z.P.; Gao, S.S.; Yue, X.A.; Lu, Y.G.; Sui, H.G.; Wei, H.G. New method for plugging channeling in oil field by nitrogen foam. J. Cent. South Univ. 2014, 21, 677–683. [Google Scholar] [CrossRef]
- Guo, Y.J.; Feng, R.S.; Zhang, X.M.; Shu, Z.; Dong, H.P.; Tang, F.M. Gelling properties of low chemical linked hydrophobically associating polymer solution in high-temperature and high-salinity reservoirs. Oil Drill. Prod. Technol. 2008, 30, 98–102. [Google Scholar]
- Chen, H.; Zhang, S.H.; Chu, Y.B.; Yang, H.X.; Liu, F.L. Preparation and use of hydrophobically associating polymer gelling fluid for water injectivity profile modification in high temperature and high salinity reservoirs. Oilfield Chem. 2004, 21, 343–346. [Google Scholar]
- Zhang, Z.L.; Zhao, L.; Cao, B.G. Applicability evaluation of hydrophobically associating polymer weak gel and application. Petrochem. Ind. Appl. 2018, 37, 65–68. [Google Scholar]
- Li, H.B.; Zhao, H.T.; Zhao, P.C.; Chen, H. Laboratory study on hydrophobically associating polymer gelling fluid as profiling agent for high temperature and high salinity reservoirs in Zhongyuan. Oilfield Chem. 2006, 23, 50–53. [Google Scholar]
- Zhou, H.T.; Huang, A.H.; Zhang, G.C.; Wu, W.M.; Fu, L.X. Research on chemical water plugging agent in high salinity reservoir at 85 °C. Oil Drill. Prod. Technol. 2009, 31, 85–89. [Google Scholar]
- Xu, Y.D.; Ge, J.J.; Song, L.F.; Zhang, Y.H.; Du, X.J. Preparation and Performance Evaluation of Cationic Polymer Chromium Gel. Oilfield Chem. 2019, 36, 230–235. [Google Scholar]
- Gaillard, N.; Giovannetti, B.; Favero, C. Improved oil recovery using thermally and chemically protected compositions based on co-and ter-polymers containing acrylamide. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Zhu, D.Y.; Hou, J.R.; Wei, Q.; Wu, X.; Bai, B.J. Terpolymer gel system formed by resorcinol–hexamethylenetetramine for water management in extremely high temperature reservoirs. Energy Fuels 2017, 31, 1519–1528. [Google Scholar] [CrossRef]
- Ye, L.; Huang, R.H. Study of P(AM-NVP-DMDA) hydrophobically associating water-soluble terpolymer. J. Appl. Polym. Sci. 1999, 74, 211–217. [Google Scholar] [CrossRef]
- Albonico, P.; Lockhart, T. Divalent ion-resistant polymer gels for high-temperature applications: Syneresis inhibiting additives. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. [Google Scholar]
- Moradi-Araghi, A. Application of low-toxicity crosslinking systems in production of thermally stable gels. In Proceedings of the SPE/DOE 9th Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–20 April 1994. [Google Scholar]
- Lockhart, T.P.; Albonico, P. New chemistry for the placement of chromium (III)/polymer gels in high-temperature reservoirs. SPE Prod. Facil. 1994, 9, 273. [Google Scholar] [CrossRef]
- Meng, Q.K.; Zhang, Y.H.; Chen, Q.Y.; Wu, Q.H.; Wei, Z.Y. Preparation of AMPS copolymer gel profile-contral agent with high thermal-resistance and salt-tolerance. Spec. Petrochem. 2017, 34, 15–19. [Google Scholar]
- Ran, Y.; Zhang, G.; Jiang, P.; Pei, H. Preparation method and performance evaluation of a gel based on AM/AMPS copolymer. Gels 2022, 8, 802. [Google Scholar] [CrossRef]
- Guo, H.; Ge, J.; Wu, Q.; He, Z.; Wang, W.; Cao, G. Syneresis behavior of polymer gels aged in different brines from gelants. Gels 2022, 8, 166. [Google Scholar] [CrossRef]
- Guo, N.; Wu, J.W.; Li, L.; Zhang, R.S.; Liao, X.A.; Chen, L.F. Study on preparation and stability mechanism of water plugging gel with high performance for Tahe oilfield. Appl. Chem. Ind. 2020, 49, 3011–3015. [Google Scholar]
- Zhang, T.C.; Ge, J.J.; Wu, H.; Guo, H.B.; Jiao, B.L.; Qian, Z. Effect of AMPS (2-acrylamido-2-methylpropane sulfonic acid) content on the properties of polymer gels. Pet. Sci. 2022, 19, 697–706. [Google Scholar] [CrossRef]
- Hsieh, H.; Moradi-Araghi, A.; Stahl, G.; Westerman, I. Watersoluble polymers for hostile environment enhanced oil recovery applications. Makromol. Chem. Macromol. Symp. 1992, 64, 121–135. [Google Scholar] [CrossRef]
- Lu, Q.X.; Yao, Y.M.; Zhao, W.M.; Yu, Q.; Pan, Z. A profile modification agent for high temperature low permeability and naturally fractured sandstone oil reservoirs and its use. Oilfield Chem. 2006, 23, 46–49. [Google Scholar]
- Hoskin, D.H.; Shu, P. Cross-Linked Polyvinyl Alcohols and Oil Reservoir Permeability Control Therewith. U.S. Patent US4859717A, 22 August 1989. [Google Scholar]
- Victorius, C. Aqueous Gel System of Partially Methylated Melamine-Formaldehyde Resin and Polyvinyl Alcohol. U.S. Patent US5061387A, 29 October 1991. [Google Scholar]
- Shu, P. High Temperature Profile Gel for Control of Oil Reservoir Permeability. U.S. Patent US5609209A, 11 March 1997. [Google Scholar]
- Vasquez, J.; Dalrymple, E.; Eoff, L.; Reddy, B.; Civan, F. Development and evaluation of high-temperature conformance polymer systems. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Dalrymple, E.D.; Everett, D.M.; Eoff, L.S. Global field results of a polymeric gel system in conformance applications. In Proceedings of the SPE Russian Oil and Gas Technical Conference and Exhibition, Moscow, Russia, 3–6 October 2006; Society of Petroleum Engineers: Richardson, TX, USA, 2006. [Google Scholar]
- Al-muntasheri, G.A.; Sierra, L.; Bakhtyarov, A. Ammonium Halide as Gelation Retarder for Crosslinkable Polymer Compositions. U.S. Patents WO2011107744A1, 9 September 2011. [Google Scholar]
- Wu, H.; Ge, J.J.; Yang, L.; Yang, Y.Y.; Zhang, T.C.; Guo, H.B. Developments of polymer gel plug for temporary blocking in SAGD wells. J. Pet. Sci. Eng. 2022, 208, 109650. [Google Scholar] [CrossRef]
- Bai, Y.R.; Xiong, C.M.; Wei, F.L.; Li, J.J.; Shu, Y.; Liu, D.X. Gelation study on a hydrophobically associating polymer/polyethylenimine gel system for water shut-off treatment. Energy Fuels 2015, 29, 447–458. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Hou, J.R.; Wei, Q.; Chen, Y.G.; Peng, K.W. Development of a high-temperature resistant polymer gel system for conformance control in Jidong Oilfield. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017. [Google Scholar]
- Albonico, P.; Lockhart, T.P. Stabilization of polymer gels against divalent ion-induced syneresis. J. Pet. Sci. Eng. 1997, 18, 61–67. [Google Scholar] [CrossRef]
- Jia, H.; Ding, M.C.; Wang, C.; Zhu, Y.R.; Liao, Y.H.; Ma, J.H.; Song, X.W.; Wang, Y.F. Development and evaluation of a seawater-based and high temperature polyacrylamide gel system. Oilfield Chem. 2022, 39, 269–275. [Google Scholar]
- Wei, Z.Y.; Dai, C.L.; Wang, C.; Zhang, Y.H.; Li, H.; Wang, K. Experimental study on thermophilic, calcium-tolerant and magnesium-tolerant profile control agents in offshore reservoir. Oilfield Chem. 2016, 33, 230–234. [Google Scholar]
- Zhang, Z.; Chen, X.R. Improving the determination of polymer hydrolysis by PH meter indicating terminal method. J. Chongqing Univ. Sci. Technol. Nat. Sci. Ed. 2015, 17, 122–124. [Google Scholar]
- Yang, W.J.; Zhao, G.; Liu, K.; Zhao, H.; Wang, P.; Dai, C.L. Experimental study on deep thermophilic and halotolerant profile control agents. Fault-Block Oil Gas Field 2011, 18, 257–260. [Google Scholar]
- Wu, Y.Q.; Bi, Y.B.; Ji, P.; Wang, J.; Jia, H.; Zhou, H.T. Water shutoff agent of PEI gel applicable to high temperature and high salinity reservoirs. Oil Drill. Prod. Technol. 2015, 37, 113–116. [Google Scholar]
- Li, C.Q.; Wang, Z.B.; Yan, G.F.; Li, H.; Song, Q. Study of HPAM/phenolic resin gelling fluid with heat resistance and salt tolerance. Adv. Fine Petrochem. 2012, 13, 4–7. [Google Scholar]
- Xu, Y.; Ge, J.J.; Guo, H.B.; Wei, K.P. The gelation law of partially hydrolyzed polyacrylamide-phenol-formaldehyde resin under medium-high temperature and medium-high salinity conditions. Acta Pet. Sin. Pet. Process. Sect. 2022, 38, 1336–1346. [Google Scholar]







| No. | Variable Factor | Temperature (°C) | Cation (Concentration in mg/L) | Gel Stability | ||
|---|---|---|---|---|---|---|
| Time (day) | Syneresis Rate (%) | Elastic Modulus (mPa) | ||||
| 1 | Temperature | 60 | Na+ (10,686) Ca2+/Mg2+ (1650) | 30 | 0.0 | 1986.0 |
| 2 | 80 | 30 | 0.0 | 1360.0 | ||
| 3 | 100 | 1 | 100.0 | – | ||
| 4 | 125 | 1 | 100.0 | – | ||
| 5 | Na+ conc. | 125 | Na+ (5000) | 30 | 0.0 | 6100.0 |
| 6 | 125 | Na+ (20,000) | 30 | 0.0 | 3860.0 | |
| 7 | 125 | Na+ (40,000) | 30 | 0.0 | 2350.0 | |
| 8 | 125 | Na+ (80,000) | 30 | 0.0 | 2120.0 | |
| 9 | Ca2+/Mg2+ conc. | 125 | Ca2+/Mg2+ (200) | 30 | 5.0 | 894.0 |
| 10 | 125 | Ca2+/Mg2+ (400) | 30 | 5.0 | 425.0 | |
| 11 | 125 | Ca2+/Mg2+ (800) | 1 | 100.0 | -- | |
| 12 | 125 | Ca2+/Mg2+ (1200) | 1 | 100.0 | -- | |
| 13 | 125 | Ca2+/Mg2+ (1600) | 1 | 100.0 | -- | |
| No. | Polymer | Gel Composition | Stability | ||
|---|---|---|---|---|---|
| Time (day) | Syneresis Rate (%) | Elastic Modulus (mPa) | |||
| HPAM | 0.8% HPAM + crosslinker | 1 | 100.0 | – | |
| 0.4% HPAM + crosslinker | 1 | 100.0 | – | ||
| PAM | 0.8% PAM + crosslinker | 1 | 100.0 | – | |
| 0.4% PAM + crosslinker | 1 | 100.0 | – | ||
| CPAM | 0.8% CPAM + crosslinker | 30 | 10.0 | 8700.0 | |
| 0.4% CPAM + crosslinker | 1 | 100.0 | – | ||
| AP-P5 | 0.8% AP-P5 + crosslinker | 30 | 10.0 | 6410.0 | |
| 0.4% AP-P5 + crosslinker | 1 | 100.0 | – | ||
| MKY | 0.8% MKY + crosslinker | 30 | 10.0 | 5530.0 | |
| 0.4% MKY + crosslinker | 2 | 100.0 | – | ||
| No. | Complexant | Gel Composition | Stability | ||
|---|---|---|---|---|---|
| Time (day) | Syneresis Rate (%) | Elastic Modulus (mPa) | |||
| 1 | DTPMP | 0.4% PAM + crosslinker + 0.3% DTPMP | 30 | 90.0 | – |
| 2 | Glycine | 0.4% PAM + crosslinker + 0.3% glycine | 30 | 20.0 | 2986.0 |
| 3 | EDTA | 0.4% PAM + crosslinker + 0.3% EDTA | 30 | 25.0 | 3206.0 |
| 4 | Sodium salicylate | 0.4% PAM + crosslinker + 0.3% sodium salicylate | 30 | 20.0 | 3010.0 |
| 5 | Sodium oxalate | 0.4% PAM + crosslinker + 0.3% sodium oxalate | 30 | 10.0 | 2960.0 |
| No. | Polymer | Gel Composition | Stability | ||
|---|---|---|---|---|---|
| Time (day) | Syneresis Rate (%) | Elastic Modulus (mPa) | |||
| 1 | HPAM | 0.8% HPAM + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 6160.0 |
| 2 | 0.4% HPAM + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 1930.0 | |
| 3 | PAM | 0.8% PAM + crosslinker +0.4% sodium oxalate | 30 | 0.0 | 8540.0 |
| 4 | 0.4% PAM + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 3230.0 | |
| 5 | CPAM | 0.8% CPAM + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 8960.0 |
| 6 | 0.4% CPAM + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 3620.0 | |
| 7 | AP-P5 | 0.8% AP-P5 + crosslinker +0.4% sodium oxalate | 30 | 0.0 | 8720.0 |
| 8 | 0.4% AP-P5 + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 3630.0 | |
| 9 | MKY | 0.8% MKY + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 7460.0 |
| 10 | 0.4% MKY + crosslinker + 0.4% sodium oxalate | 30 | 0.0 | 2560.0 | |
| No. | Polymer | Gel Composition | Stability | |||
|---|---|---|---|---|---|---|
| Time (day) | Syneresis Rate (%) | Elastic Modulus (mPa) | ||||
| 1 | SAV10 | 0.4% SAV10 + crosslinker | 30 | 0.0 | 106.0 | |
| 2 | 0.8% SAV10 + crosslinker | 30 | 0.0 | 361.0 | ||
| 3 | SAV55 | 0.4% SAV55 + crosslinker | 30 | 0.0 | 469.0 | |
| 4 | 0.8% SAV55 + crosslinker | 30 | 0.0 | 1860.0 | ||
| Type | Abbreviation | Molecular Weight (×104) | Hydrolysis Degree (%) | Supplier |
|---|---|---|---|---|
| Anionic | HPAM | 1600 | 21.3 | Hebei Xinxing Chemical Co., Ltd. |
| Nonionic | PAM | 1600 | 3.1 | |
| Cationic | CPAM | 1600 | 1.4 | |
| Hydrophobic association | AP-P5 | 1600 | - | Sichuan Guangya Polymer Co., Ltd. |
| MKY | 1600 | - | ||
| Anionic | SAV10 | 300–500 | 6.0 | Ethan Co., France (SNF) |
| SAV55 | 6.0 |
| No. | Ion Type/Concentration (mg/L) | TDS (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| K+ + Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | ||
| 1 | 10,686.0 | 439.0 | 1211.0 | 19,457.0 | 1619.0 | 226.0 | 33,645.0 |
| 2 | 77,974.0 | 9410.0 | 1462.0 | 140,257.0 | 736.0 | 337.0 | 225,068.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Ding, M.; Hu, Y.; Wang, Y.; Dong, J. Optimization of the Methods to Develop Stable Polymer Gels for Water Management in Medium- and Ultra-High-Salinity Reservoirs. Gels 2023, 9, 540. https://doi.org/10.3390/gels9070540
Hu S, Ding M, Hu Y, Wang Y, Dong J. Optimization of the Methods to Develop Stable Polymer Gels for Water Management in Medium- and Ultra-High-Salinity Reservoirs. Gels. 2023; 9(7):540. https://doi.org/10.3390/gels9070540
Chicago/Turabian StyleHu, Shuiqing, Mingchen Ding, Yafei Hu, Yefei Wang, and Jiangyang Dong. 2023. "Optimization of the Methods to Develop Stable Polymer Gels for Water Management in Medium- and Ultra-High-Salinity Reservoirs" Gels 9, no. 7: 540. https://doi.org/10.3390/gels9070540