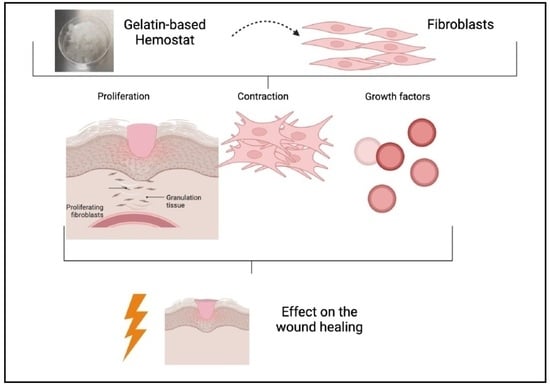
Effect of Gelatin-Based Hemostats on Fibroblasts and Relevant Growth Factors in Wound Healing
Abstract
:1. Introduction
2. Results
2.1. Cell Proliferation
2.2. Contraction Assay
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
3. Discussion
3.1. Cell Proliferation
3.2. Cell Contraction
3.3. Growth Factors
4. Conclusions
5. Material and Methods
5.1. Hemostat GELITA TUFT-IT®
5.2. Cell Culture
5.3. Cell Proliferation
5.4. Contraction Assay
5.5. Enzyme-Linked Immunosorbent Assay (ELISA)
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FGF-b | Basic fibroblast growth factor |
| DMRM | Dulbecco’s phosphate-buffered saline |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| NHDF | Normal human dermal fibroblast |
| PBS | Phosphate buffered saline |
| PDGF | Platelet-derived growth factor |
| TGFβ | Transforming growth factor β |
| TNFα | Tumor necrosis factor α |
| SEM | Standard error of the mean |
| VEGF | Vascular endothelial growth factor |
References
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Shams, F.; Moravvej, H.; Hosseinzadeh, S.; Mostafavi, E.; Bayat, H.; Kazemi, B.; Bandehpour, M.; Rostami, E.; Rahimpour, A.; Moosavian, H. Overexpression of VEGF in dermal fibroblast cells accelerates the angiogenesis and wound healing function: In vitro and in vivo studies. Sci. Rep. 2022, 12, 18529. [Google Scholar] [CrossRef]
- Desmouliere, A.; Redard, M.; Darby, I.; Gabbiani, G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995, 146, 56–66. [Google Scholar] [PubMed]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Oberringer, M.; Meins, C.; Bubel, M.; Pohlemann, T. In vitro wounding: Effects of hypoxia and transforming growth factor beta1 on proliferation, migration and myofibroblastic differentiation in an endothelial cell-fibroblast co-culture model. J. Mol. Histol. 2008, 39, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef] [Green Version]
- Welch, M.P.; Odland, G.F.; Clark, R.A. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J. Cell Biol. 1990, 110, 133–145. [Google Scholar] [CrossRef]
- Vedrenne, N.; Coulomb, B.; Danigo, A.; Bonte, F.; Desmouliere, A. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol. Biol. 2012, 60, 20–27. [Google Scholar] [CrossRef]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, N.W.; Yancopoulos, G.D. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999, 13, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Lawrence, W.T.; Diegelmann, R.F. Growth factors in wound healing. Clin. Dermatol. 1994, 12, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wada, R.; Yamashita, T.; Mi, Y.; Deng, C.X.; Hobson, J.P.; Rosenfeldt, H.M.; Nava, V.E.; Chae, S.S.; Lee, M.J.; et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000, 106, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Watterson, K.R.; Lanning, D.A.; Diegelmann, R.F.; Spiegel, S. Regulation of fibroblast functions by lysophospholipid mediators: Potential roles in wound healing. Wound Repair. Regen. 2007, 15, 607–616. [Google Scholar] [CrossRef]
- Wagenhauser, M.U.; Mulorz, J.; Ibing, W.; Simon, F.; Spin, J.M.; Schelzig, H.; Oberhuber, A. Oxidized (non)-regenerated cellulose affects fundamental cellular processes of wound healing. Sci. Rep. 2016, 6, 32238. [Google Scholar] [CrossRef] [Green Version]
- Wagenhauser, M.U.; Garabet, W.; van Bonn, M.; Ibing, W.; Mulorz, J.; Rhee, Y.H.; Spin, J.M.; Dimopoulos, C.; Oberhuber, A.; Schelzig, H.; et al. Time-dependent effects of cellulose and gelatin-based hemostats on cellular processes of wound healing. Arch. Med. Sci. 2023, 19, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Charlesworth, T.M.; Agthe, P.; Moores, A.; Anderson, D.M. The use of haemostatic gelatin sponges in veterinary surgery. J. Small Anim. Pract. 2012, 53, 51–56. [Google Scholar] [CrossRef]
- Chvapil, M.; Owen, J.A.; DeYoung, D.W. A standardized animal model for evaluation of hemostatic effectiveness of various materials. J. Trauma Acute Care Surg. 1983, 23, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Coln, D.; Horton, J.; Ogden, M.E.; Buja, L.M. Evaluation of hemostatic agents in experimental splenic lacerations. Am. J. Surg. 1983, 145, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.; Oyaguez, I.; Casado, M.A. Use of Floseal(R), a human gelatine-thrombin matrix sealant, in surgery: A systematic review. BMC Surg. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajosch, R.; Suckfuell, M.; Oesser, S.; Ahlers, M.; Flechsenhar, K.; Schlosshauer, B. A novel gelatin sponge for accelerated hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.P.; Senz, E.H.; Owen, H.W.; Jampolis, R.W. Present status of gelatin sponge for the control of hemorrhage; with experimental data on its use for wounds of the great vessels and the heart. J. Am. Med. Assoc. 1946, 132, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Na, Y.C.; Jin, Y.W. Comparison of the wound healing effect of cellulose and gelatin: An in vivo study. Arch. Plast. Surg. 2012, 39, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, L.K.; Mohanty, M.; Umashankar, P.R.; Lal, A.V. Comparative evaluation of absorbable hemostats: Advantages of fibrin-based sheets. Biomaterials 2004, 25, 5557–5563. [Google Scholar] [CrossRef]
- Moscato, S.; Mattii, L.; D’Alessandro, D.; Cascone, M.G.; Lazzeri, L.; Serino, L.P.; Dolfi, A.; Bernardini, N. Interaction of human gingival fibroblasts with PVA/gelatine sponges. Micron 2008, 39, 569–579. [Google Scholar] [CrossRef]
- Ozer, A.; Kostu, B. Use of Gelatin Sponge Affects Postoperative Morbidity In Cesarean Section Patients. Med. Sci. Monit. 2017, 23, 1141–1145. [Google Scholar] [CrossRef] [Green Version]
- Sener, I.; Bereket, C.; Arslan, G.; Ozkan, N.; Ozdemir, M.; Mercan, U.; Ayyildiz, M.; Agar, E. The effect of hemostatic agents and tissue adhesive on injured peripheral nerve healing in rats—Part I. Electrophysiological study. Adv. Clin. Exp. Med. 2015, 24, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Miyahara, J.; Matsubayashi, Y.; Taniguchi, Y.; Doi, T.; Kodama, H.; Higashikawa, A.; Takeshita, Y.; Fukushima, M.; Ono, T.; et al. Predictors for hemostatic thrombin-gelatin matrix usage in spine surgery: A multicenter observational study. BMC Musculoskelet. Disord. 2023, 24, 289. [Google Scholar] [CrossRef] [PubMed]
- Liesegang, J.F. Die Gelatine in der Medizin. Ph.D. Thesis, Medizinische Fakultät Heidelberg, Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Sundaram, C.P.; Keenan, A.C. Evolution of hemostatic agents in surgical practice. Indian J. Urol. 2010, 26, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.S.; Saha, S.P. Comparison of hemostatic agents used in vascular surgery. Expert Opin. Biol. Ther. 2013, 13, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Zoucas, E.; Goransson, G.; Bengmark, S. Comparative evaluation of local hemostatic agents in experimental liver trauma: A study in the rat. J. Surg. Res. 1984, 37, 145–150. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, L.; Huang, Y.; Chen, M.; Li, M.; Cai, K.; Luo, Z.; Hu, Y. Multifunctional antibiotics-free hydrogel dressings with self-regulated nitric oxide-releasing kinetics for improving open wound healing. J. Mater. Chem. B 2023, 11, 3650–3668. [Google Scholar] [CrossRef]
- Valencia-Gomez, L.E.; Reyes-Blas, H.; Hernandez-Paz, J.F.; Rodriguez-Gonzalez, C.A.; Olivas-Armendariz, I. Comparative Study of the Antibacterial, Biodegradable, and Biocompatibility Properties of Composite and Bi-Layer Films of Chitosan/Gelatin Coated with Silver Particles. Materials 2023, 16, 3000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, X.; He, W.; Li, H.; Huang, Y.; Wu, G. Autocatalytically hydroxyl-producing composite wound dressing for bacteria-infected wound healing. Nanomedicine 2023, 51, 102683. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yuan, X.; Wu, Z.; Park, S.; Zhang, W.; Chong, H.; Lin, L.; Piao, Y. Fabrication and Performance Evaluation of Gelatin/Sodium Alginate Hydrogel-Based Macrophage and MSC Cell-Encapsulated Paracrine System with Potential Application in Wound Healing. Int. J. Mol. Sci. 2023, 24, 1240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sakamoto, M.; Matsuno, K.; Sawaragi, E.; Zhao, Q.; Nakano, T.; Yamanaka, H.; Tsuge, I.; Katayama, Y.; Shimada, N.; et al. Modified gelatin hydrogel nonwoven fabrics (Genocel) as a skin substitute in murine skin defects. Regen. Ther. 2023, 23, 44–51. [Google Scholar] [CrossRef]
- Orlova, A.A.; Kotlyarova, M.S.; Lavrenov, V.S.; Volkova, S.V.; Arkhipova, A.Y. Relationship between gelatin concentrations in silk fibroin-based composite scaffolds and adhesion and proliferation of mouse embryo fibroblasts. Bull. Exp. Biol. Med. 2014, 158, 88–91. [Google Scholar] [CrossRef]
- Zeng, Q.O.; Chen, W.L. The functional behavior of a macrophage/fibroblast co-culture model derived from normal and diabetic mice with a marine gelatin oxidized alginate hydrogel. Biomaterials 2010, 31, 5772–5781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS ONE 2014, 9, e0107952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Gupta, A.; Agrawal, A.K.; Jassal, M.; Dinda, A.K.; Koul, V. Bi-Layer Composite Dressing of Gelatin Nanofibrous Mat and Poly Vinyl Alcohol Hydrogel for Drug Delivery and Wound Healing Application: In-Vitro and In-Vivo Studies. J. Biomed. Nanotechnol. 2013, 9, 1495–1508. [Google Scholar] [CrossRef]
- Nakajima, K.; Fujita, J.; Matsui, M.; Tohyama, S.; Tamura, N.; Kanazawa, H.; Seki, T.; Kishino, Y.; Hirano, A.; Okada, M.; et al. Gelatin Hydrogel Enhances the Engraftment of Transplanted Cardiomyocytes and Angiogenesis to Ameliorate Cardiac Function after Myocardial Infarction. PLoS ONE 2015, 10, e0133308. [Google Scholar] [CrossRef] [Green Version]
- Schiefer, J.L.; Rath, R.; Held, M.; Petersen, W.; Werner, J.O.; Schaller, H.E.; Rahmanian-Schwarz, A. Frequent Application of the New Gelatin-Collagen Nonwoven Accelerates Wound Healing. Adv. Ski. Wound Care 2016, 29, 73–78. [Google Scholar] [CrossRef]
- Cahu, T.B.; Silva, R.A.; Silva, R.P.F.; Silva, M.M.; Arruda, I.R.S.; Silva, J.F.; Costa, R.M.P.B.; Santos, S.D.; Nader, H.B.; Bezerra, R.S. Evaluation of Chitosan-Based Films Containing Gelatin, Chondroitin 4-Sulfate and ZnO for Wound Healing. Appl. Biochem. Biotechnol. 2017, 183, 765–777. [Google Scholar] [CrossRef]
- Nagelschmidt, M.; Fischer, H.; Engelhardt, G.H. Reversal of Gelatin-Impaired Wound-Healing in Rats by Exogenous Fibronectin. J. Surg. Res. 1992, 53, 490–494. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Huda, F.; Pant, J.; Ghosh Kar, A.; Banerjee, T.; Shukla, V.K. An appraisal of vascular endothelial growth factor (VEGF): The dynamic molecule of wound healing and its current clinical applications. Growth Factors 2022, 40, 73–88. [Google Scholar] [CrossRef]
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef]
- Morbidelli, L.; Chang, C.H.; Douglas, J.G.; Granger, H.J.; Ledda, F.; Ziche, M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am. J. Physiol. 1996, 270 Pt 2, H411–H415. [Google Scholar] [CrossRef]
- Kawai, K.; Suzuki, S.; Tabata, Y.; Ikada, Y.; Nishimura, Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 2000, 21, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Morimoto, N.; Ikada, Y. Gelatin gel as a carrier of platelet-derived growth factors. J. Biomater. Appl. 2013, 28, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Jinno, C.; Morimoto, N.; Ito, R.; Sakamoto, M.; Ogino, S.; Taira, T.; Suzuki, S. A Comparison of Conventional Collagen Sponge and Collagen-Gelatin Sponge in Wound Healing. Biomed. Res. Int. 2016, 2016, 4567146. [Google Scholar] [CrossRef] [Green Version]
- Kakudo, N.; Morimoto, N.; Ogawa, T.; Kusumoto, K. Effects of Fibroblast Growth Factor-2 Combined With a Collagen/Gelatin Sponge for Adipogenesis in the Mouse Subcutis. Ann. Plast. Surg. 2020, 84, 216–221. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garabet, W.; Shabes, P.; Wolters, K.H.; Rembe, J.-D.; Ibing, W.; Wagenhäuser, M.U.; Simon, F.; Schelzig, H.; Oberhuber, A. Effect of Gelatin-Based Hemostats on Fibroblasts and Relevant Growth Factors in Wound Healing. Gels 2023, 9, 504. https://doi.org/10.3390/gels9060504
Garabet W, Shabes P, Wolters KH, Rembe J-D, Ibing W, Wagenhäuser MU, Simon F, Schelzig H, Oberhuber A. Effect of Gelatin-Based Hemostats on Fibroblasts and Relevant Growth Factors in Wound Healing. Gels. 2023; 9(6):504. https://doi.org/10.3390/gels9060504
Chicago/Turabian StyleGarabet, Waseem, Polina Shabes, Katharina Henrika Wolters, Julian-Dario Rembe, Wiebke Ibing, Markus Udo Wagenhäuser, Florian Simon, Hubert Schelzig, and Alexander Oberhuber. 2023. "Effect of Gelatin-Based Hemostats on Fibroblasts and Relevant Growth Factors in Wound Healing" Gels 9, no. 6: 504. https://doi.org/10.3390/gels9060504