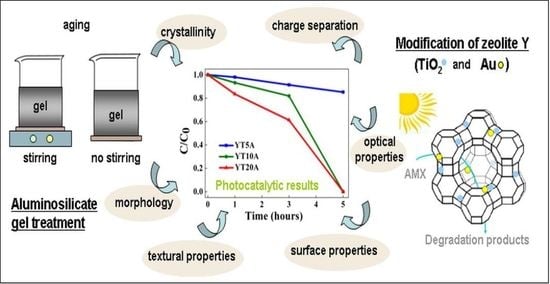
Effects of Aluminosilicate Gel Treatment and TiO2 Loading on Photocatalytic Properties of Au–TiO2/Zeolite Y
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Aluminosilicate Gel Treatment
2.1.1. XRD Analysis
2.1.2. Raman Spectroscopy
2.1.3. SEM Images
2.2. Effect of TiO2 Loading
2.2.1. XRD Analysis
2.2.2. Textural Analysis
2.2.3. SEM Images
2.2.4. XPS Results
2.2.5. Raman Spectroscopy
2.2.6. UV–Vis Absorbance Spectroscopy
2.2.7. Photoluminescence Spectroscopy
2.2.8. H2-TPR Studies
2.2.9. CO2-TPD Results
2.2.10. Adsorption Studies
2.2.11. Photocatalytic Activity
2.2.12. Kinetic Studies
2.2.13. Toxicity Assessment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Chemicals
4.1.2. Sample Preparation
4.2. Methods of Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornas, A.; Olszówka, J.E.; Urbanova, M.; Brabec, L.; Rathousky, J.; Dedecek, J.; Pashkova, V. Ultrasonic Pretreatment as a Tool for the Preparation of Low-Defect Zeolite Mordenite. ACS Omega. 2021, 6, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, F.; Bengueddach, A.; Di Renzo, F.; Fajula, F. Control of Crystal Size and Morphology of Mordenite. Catal. Lett. 2003, 87, 149–152. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T.; Wang, J.; Fan, W. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W. Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef] [Green Version]
- Yu, J. Synthesis of zeolites. In Introduction to Zeolite Science and Practice, 3rd ed.; Cejka, J., van Bekkum, H., Corma, A., Schuth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 40–103. [Google Scholar]
- Munguti, L.K.; Dejene, F.B.; Muthee, D.K. Zeolite Na-A supported TiO2: Effects of TiO2 loading on structural, optical and adsorption properties. Mater. Sci. Eng. B 2023, 289, 116281. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Sánchez-Rubio, M.; Navlani-García, M.; Berenguer-Murcia, A.; Cazorla-Amorós, D. Synthesis of TiO2/Nanozeolite Composites for Highly Efficient Photocatalytic Oxidation of Propene in the Gas Phase. ACS Omega 2020, 5, 31323–31331. [Google Scholar] [CrossRef]
- Joseph, C.G.; Sharain-Liew, Y.L.; Bono, A.; Teng, L.Y. Photodegradation of Indigo Dye Using TiO2 and TiO2/Zeolite System. Asian J. Chem. 2013, 25, 8402–8406. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Ren, F.; Shuai, H.; Du, G. Nonmetallic Mineral as the Carrier of TiO2 Photocatalyst: A Review. Front. Catal. 2022, 2, 806316. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Chang Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Awad, M.E.; Farrag, A.M.; El-Bindary, A.A.; El-Bindary, M.A.; Kiwaan, H.A. Photocatalytic degradation of Rhodamine B dye using lowcost pyrofabricated titanium dioxide quantum dotskaolinite nanocomposite. Appl. Organomet. Chem. 2023, e7113. [Google Scholar] [CrossRef]
- Preda, S.; Pandele-Cusu, J.; Petrescu, S.V.; Ciobanu, E.M.; Petcu, G.; Culita, D.C.; Apostol, N.G.; Costescu, R.M.; Raut, I.; Constantin, M.; et al. Photocatalytic and Antibacterial Properties of Doped TiO2 Nanopowders Synthesized by Sol−Gel Method. Gels 2022, 8, 673. [Google Scholar] [CrossRef]
- Predoana, L.; Petcu, G.; Preda, S.; Pandele-Cus, U.J.; Petrescu, S.V.; Baran, A.; Apostol, N.G.; Costescu, R.M.; Surdu, V.-A.; Vasile, B.Ş.; et al. Copper-/Zinc-Doped TiO2 Nanopowders Synthesized by Microwave-Assisted Sol–Gel Method. Gels 2023, 9, 267. [Google Scholar] [CrossRef]
- Jaybhaye, S.; Shinde, N.; Jaybhaye, S.; Narayan, H. Photocatalytic Degradation of Organic Dyes Using Titanium Dioxide (TiO2) and Mg-TiO2 Nanoparticles. J. Nanotechnol. Nanomater. 2022, 3, 67–76. [Google Scholar] [CrossRef]
- Predoană, L.; Ciobanu, E.M.; Petcu, G.; Preda, S.; Pandele–Cușu, J.; Anghel, E.M.; Petrescu, S.V.; Culiță, D.C.; Băran, A.; Surdu, V.-A.; et al. Photocatalytic Performance of Sn–Doped TiO2 Nanopowders for Photocatalytic Degradation of Methyl Orange Dye. Catalysts 2023, 13, 534. [Google Scholar] [CrossRef]
- Usman, A.K.; Cursaru, D.-L.; Branoiu, G.; Somoghi, R.; Manta, A.-M.; Matei, D.; Mihai, S. A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels 2022, 8, 728. [Google Scholar] [CrossRef]
- Park, B.-G. Photocatalytic Activity of TiO2 -Doped Fe, Ag, and Ni with N under Visible Light Irradiation. Gels 2022, 8, 14. [Google Scholar] [CrossRef]
- Khusnun, N.F.; Jalil, A.A.; Abdullah, T.A.T.; Latip, S.S.M.; Hitam, C.N.C.; Fauzi, A.A.; Hassan, N.S.; Aziz, M.A.H.; Rahman, A.F.A.; Aziz, F.F.A.; et al. Influence of TiO2 dispersion on silica support toward enhanced amine assisted CO2 photoconversion to methanol. J. CO2 Util. 2022, 58, 101901. [Google Scholar] [CrossRef]
- Petcu, G.; Anghel, E.M.; Buixaderas, E.; Atkinson, I.; Somacescu, S.; Baran, A.; Culita, D.C.; Trica, B.; Bradu, C.; Ciobanu, M.; et al. Au/Ti Synergistically Modified Supports Based on SiO2 with Different Pore Geometries and Architectures. Catalysts 2022, 12, 1129. [Google Scholar] [CrossRef]
- Danfá, S.; Oliveira, C.; Santos, R.; Martins, R.C.; Quina, M.M.J.; Gomes, J. Development of TiO2-Based Photocatalyst Supported on Ceramic Materials for Oxidation of Organic Pollutants in Liquid Phase. Appl. Sci. 2022, 12, 7941. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Omri, A.; Zina, B.B.; Da Costa, P.; Galvez, M.E. Titanium Dioxide Supported on Different Porous Materials as Photocatalyst for the Degradation of Methyl Green in Wastewaters. Adv. Mater. Sci. Eng. 2015, 2015, 759853. [Google Scholar] [CrossRef] [Green Version]
- Kucerová, G.; Strunk, J.; Muhler, M.; Behm, R.J. Effect of titania surface modification of mesoporous silica SBA-15 supported Au catalysts: Activity and stability in the CO oxidation reaction. J. Catal. 2017, 356, 214–228. [Google Scholar] [CrossRef]
- Liao, G.; He, W.; He, Y. Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO₂ Composite. Catalysts 2019, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Manttari, M.; Sillanpaa, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Yu, H.; Li, S.; Peng, S.; Yu, Z.; Chen, F.; Liu, X.; Guo, J.; Zhu, B.; Huang, W.; Zhang, S. Construction of rutile/anatase TiO2 homojunction and metal-support interaction in Au/TiO2 for visible photocatalytic water splitting and degradation of methylene blue. Int. J. Hydrog. Energ. 2023, 48, 975–990. [Google Scholar] [CrossRef]
- Zerjav, G.; Roskari, M.; Zavasnik, J.; Kovac, J.; Pintar, A. Effect of Au loading on Schottky barrier height in TiO2 + Au plasmonic photocatalysts. Appl. Surf. Sci. 2022, 579, 152196. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Wu, S.; Lian, H.; Deng, X. The exceptional performance of the plasmonic Au-Fe/TiO2 nanocatalysts achieved by O2 plasma activation. Catal. Today 2023, 418, 114106. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Li, X.-S.; Liu, J.-L.; Li, Y.-C.; Zhu, B.; Zhu, A.-M. A promising visible-light photocatalyst: H2 plasma-activated amorphous-TiO2-supported Au nanoparticles. J. Catal. 2019, 375, 380–388. [Google Scholar] [CrossRef]
- Petcu, G.; Dobrescu, G.; Atkinson, I.; Ciobanu, M.; Blin, J.-L.; Parvulescu, V. Evolution of Morphology, Fractal Dimensions, and Structure of (Titanium) Aluminosilicate Gel during Synthesis of Zeolites Y and Ti-Y. Fractal Fract. 2022, 6, 663. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 154–155. [Google Scholar]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F.S. Characterization of aluminosilicate zeolites by UV Raman spectroscopy. Micropor. Mesopor. Mater. 2001, 46, 23–34. [Google Scholar] [CrossRef]
- Kishor, R.; Singh, S.B.; Ghoshal, A.K. Role of metal type on mesoporous KIT-6 for hydrogen storage. Int. J. Hydrog. Energ. 2018, 43, 10376–10385. [Google Scholar] [CrossRef]
- Petcu, G.; Anghel, E.M.; Somacescu, S.; Preda, S.; Culita, D.; Mocanu, S.; Ciobanu, M.; Parvulescu, V. Hierarchical Zeolite Y Containing Ti and Fe Oxides as Photocatalysts for Degradation of Amoxicillin. J. Nanosci. Nanotechno. 2020, 20, 1158–1169. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Estevez, R.; Hidalgo-Carrillo, J.; Lopez-Fernandez, S.; Urbano, F.J.; Marinas, A. Hydrogen photo-production from glycerol on platinum, gold and silver-modified TiO2-USY62 catalysts. Catal. Today 2022, 390–391, 92–98. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Poell, C.J. NIST X-Ray Photoelectron Spectroscopy Database, Version 4.1. NIST Standard Reference Database NIST SRD 20. National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Moulder, F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; ULVAC-PHI, Inc.: Chigasaki, Japan; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995; p. 370. [Google Scholar]
- Petcu, G. Performing Catalytic Materials Based on Zeolite Y and Mesoporous Silica, Functionalized with Transition Metals, Used in Degradation and Recovery of Different Pollutants from Water and Air. Ph.D. Thesis, School of Advanced Studies of the Romanian Academy, Bucharest, Romania, 26 October 2021. [Google Scholar]
- Perera, A.S.; Trogadas, P.; Nigra, M.M.; Yu, H.; Coppens, M.O. Optimization of mesoporous titanosilicate catalysts for cyclohexene epoxidation via statistically guided synthesis. J. Mater. Sci. 2018, 53, 7279–7293. [Google Scholar] [CrossRef] [Green Version]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Li, M.; Feng, Z.; Li, C. UV Raman Spectroscopic Study on TiO2. II. Effect of Nanoparticle Size on the Outer/Inner Phase Transformations. J. Phys. Chem. C 2009, 113, 1698–1704. [Google Scholar] [CrossRef]
- Peng, R.; Zhao, D.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Room Temperature Synthesis of Ti–MCM-48 and Ti–MCM-41 Mesoporous Materials and Their Performance on Photocatalytic Splitting of Water. J. Phys. Chem. C. 2012, 116, 1605–1613. [Google Scholar] [CrossRef]
- Sacaliuc, E.; Beale, A.M.; Weckhuysen, B.M.; Nijhuis, T.A. Propene epoxidation over Au/Ti-SBA-15 catalysts. J. Catal. 2007, 248, 235–248. [Google Scholar] [CrossRef]
- Jianga, C.; Lee, K.Y.; Parlett, C.M.A.; Bayazit, M.K.; Lau, C.C.; Ruan, Q.; Moniz, S.J.A.; Lee, A.F.; Tang, J. Size-controlled TiO2 nanoparticles on porous hosts for enhanced photocatalytic hydrogen production. Appl. Catal. A Gen. 2016, 521, 133–139. [Google Scholar] [CrossRef]
- Yadav, R.; Amoli, V.; Singh, J.; Tripathi, M.K.; Bhanja, P.; Bhaumik, A.; Sinha, A.K. Plasmonic gold deposited on mesoporous TixSi1−xO2 with isolated silica in lattice: An excellent photocatalyst for photocatalytic conversion of CO2 into methanol under visible light irradiation. J. CO2 Util. 2018, 27, 11–21. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, C.; Lv, Y.; Gao, F.; Dong, L. Direct synthesis of Ti-SBA-15 in the self-generated acidic environment and its photodegradation of Rhodamine. J. Porous Mater. 2014, 21, 63–70. [Google Scholar] [CrossRef]
- Parvulescu, V.; Ciobanu, M.; Petcu, G. Immobilization of semiconductor photocatalysts. In Handbook of Smart Photocatalytic Materials Fundamentals, Fabrications, and Water Resources Applications; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–140. [Google Scholar] [CrossRef]
- Zhu, Q.; Lu, J.; Wang, Y.; Qin, F.; Shi, Z.; Xu, C. Burstein-Moss Effect behind Au Surface Plasmon Enhanced Intrinsic Emission of ZnO Microdisks. Sci. Rep. 2016, 6, 36194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaziz, N.; Ben Manaa, M.; Aouaini, F.; Ben Lamine, A. Investigation of hydrogen adsorption on zeolites A, X and Y using statistical physics formalism. Materi. Chem. Phys. 2019, 225, 111–121. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, K.Q.; Yu, J.; Xu, B.Q. A key to the storage stability of Au/TiO2 catalyst. Phys. Chem. Chem. Phys. 2008, 10, 6399–6404. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, B.; Li, G.; Li, Y.C. N co-doping promoted mesoporous Au/TiO2 catalyst for low temperature CO oxidation. RSC Adv. 2016, 6, 28904–28911. [Google Scholar] [CrossRef]
- Chong, C.C.; Teh, L.P.; Setiabudi, H.D. Syngas production via CO2 reforming of CH4 over Ni-based SBA-15: Promotional effect of promoters (Ce, Mg, and Zr). Mater. Today Energy 2019, 12, 408–417. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Aouad, S.; El-Ayadi, H.; Skaf, M.; Labaki, M.; Cousin, R.; Abi-Aad, E. Physicochemical characterization of Au/CeO2 solids. Part 2: The impregnation preparation method. Mater. Chem. Phys. 2012, 137, 42–47. [Google Scholar] [CrossRef]
- Shin, H.; Choi, M.; Kim, H. A mechanistic model for hydrogen activation, spillover, and its chemical reaction in a zeolite-encapsulated Pt catalyst. Phys. Chem. Chem. Phys. 2016, 18, 7035–7041. [Google Scholar] [CrossRef]
- Im, J.; Shin, H.; Jang, H.; Kim, H.; Choi, M. Maximizing the catalytic function of hydrogen spillover in platinum-encapsulated aluminosilicates with controlled nanostructures. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Barakat, T.; Idakiev, V.; Cousin, R.; Shao, G.S.; Yuan, Z.Y.; Tabakova, T.; Siffert, S. Total oxidation of toluene over noble metal based Ce, Fe and Ni doped titanium oxides. Appl. Catal. B. 2014, 146, 138–146. [Google Scholar] [CrossRef]
- Chinh, V.D.; Broggi, A.; Di Palma, L.; Scarsella, M.; Speranza, G.; Vilardi, G.; Thang, P.N. XPS Spectra Analysis of Ti2+, Ti3+ Ions and Dye Photodegradation Evaluation of Titania-Silica Mixed Oxide Nanoparticles. J. Electron. Mater. 2018, 47, 2215–2224. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Al-Qahtani, S.D.; Alshareef, M.; El-Desouky, M.G.; El-Bindary, A.A.; El-Metwaly, N.M.; El-Bindary, M.A. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process Saf. Environ. Prot. 2023, 172, 395–407. [Google Scholar] [CrossRef]
- Liu, W.; He, T.; Wang, Y.; Ning, G.; Xu, Z.; Chen, X.; Hu, X.; Wu, Y.; Zhao, Y. Synergistic adsorption-photocatalytic degradation efect and norfoxacin mechanism of ZnO/ZnS@BC under UV-light irradiation. Sci. Rep. 2020, 10, 11903. [Google Scholar] [CrossRef]
- Zamani, F.; Rezapour, M.; Kianpour, S. Immobilization of L-lysine on Zeolite 4A as an Organic-Inorganic Composite Basic Catalyst for Synthesis of α,β-Unsaturated Carbonyl Compounds under Mild Conditions. Bull. Korean Chem. Soc. 2013, 34, 2367. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Yuan, X.; Yao, Q.; Xie, J. Shining photocatalysis by gold-based nanomaterials. Nano Energy 2021, 88, 106306. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, R.; Hu, K. Gold nanoclusters: Photophysical properties and photocatalytic applications. Front. Chem. 2022, 10, 958626. [Google Scholar] [CrossRef]
- Yu, C.; Li, G.; Kumar, S.; Kawasaki, H.; Jin, R. Stable Au25(SR)18/TiO2 Composite Nanostructure with Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. Lett. 2013, 4, 2847–2852. [Google Scholar] [CrossRef]
- Lee, J.; Shim, H.S.; Lee, M.; Song, J.K.; Lee, D. Size-Controlled Electron Transfer and Photocatalytic Activity of ZnO-Au Nanoparticle Composites. J. Phys. Chem. Lett. 2011, 2, 2840–2845. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of a Thiol-Protected Au25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Kogo, A.; Sakai, N.; Tatsuma, T. Photoelectrochemical analysis of size- dependent electronic structures of gold clusters supported on TiO2. Nanoscale 2012, 4, 4217–4221. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Q.; Han, C.; Yang, Y.; Jiang, W.; Zhang, Z. Photocatalytic degradation of recalcitrant organic pollutants in water using a novel cylindrical multi-column photoreactor packed with TiO2-coated silica gel beads. J. Hazard. Mater. 2015, 285, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]




















| Sample | BET Surface Area (m2/g) | Vpore (cm3/g) | Pore Size (nm) | TiO2 Crystallite Size (nm) | Band Gap Energy (eV) |
|---|---|---|---|---|---|
| YT5 | 850 | 0.102 | 1.6 | 9.9 | 3.09 |
| YT5A | 856 | 0.104 | 1.6 | 10.0 | 3.22 |
| YT10 | 678 | 0.107 | 1.8 | 10.2 | 3.17 |
| YT10A | 699 | 0.109 | 1.8 | 10.3 | 3.20 |
| YT20 | 507 | 0.114 | 2.0 | 12.1 | 3.14 |
| YT20A | 572 | 0.154 | 2.0 | 12.3 | 3.15 |
| Sample | Binding Energy (eV) | Au Chemical Species Rel. Conc. | ||||||
|---|---|---|---|---|---|---|---|---|
| Au4f/2 Metallic nps | Au4f/2 Clusters | Au4f/2 Au+ | Au4f/2 Au3+ | Au Metallic nps | Clusters | Au1+ | Au3+ | |
| YT5A | 83.3 | 84.3 | 85.3 | 86.7 | 56.1 | - | - | 43.9 |
| YT10A | 83.3 | 84.3 | 85.3 | 86.7 | 44.7 | 18.8 | 18 | 18.6 |
| YT20A | 83.3 | 84.3 | 85.3 | 86.7 | 45.7 | 19.2 | 16 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petcu, G.; Papa, F.; Anghel, E.M.; Atkinson, I.; Preda, S.; Somacescu, S.; Culita, D.C.; Baran, A.; Ciobanu, E.M.; Jecu, L.M.; et al. Effects of Aluminosilicate Gel Treatment and TiO2 Loading on Photocatalytic Properties of Au–TiO2/Zeolite Y. Gels 2023, 9, 503. https://doi.org/10.3390/gels9060503
Petcu G, Papa F, Anghel EM, Atkinson I, Preda S, Somacescu S, Culita DC, Baran A, Ciobanu EM, Jecu LM, et al. Effects of Aluminosilicate Gel Treatment and TiO2 Loading on Photocatalytic Properties of Au–TiO2/Zeolite Y. Gels. 2023; 9(6):503. https://doi.org/10.3390/gels9060503
Chicago/Turabian StylePetcu, Gabriela, Florica Papa, Elena Maria Anghel, Irina Atkinson, Silviu Preda, Simona Somacescu, Daniela C. Culita, Adriana Baran, Elena Madalina Ciobanu, Luiza Maria Jecu, and et al. 2023. "Effects of Aluminosilicate Gel Treatment and TiO2 Loading on Photocatalytic Properties of Au–TiO2/Zeolite Y" Gels 9, no. 6: 503. https://doi.org/10.3390/gels9060503