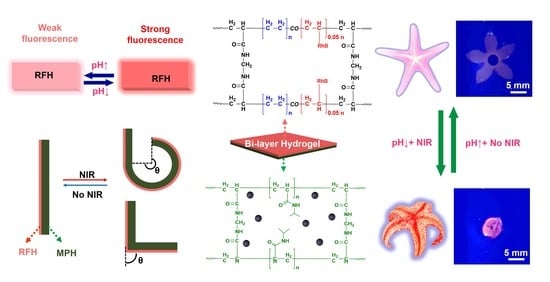
Anisotropic Bi-Layer Hydrogel Actuator with pH-Responsive Color-Changing and Photothermal-Responsive Shape-Changing Bi-Functional Synergy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of the Bi-Layer Anisotropic Hydrogel
2.2. pH-Responsive Fluorescent Color-Changing Performance of the RFH LAYER
2.3. Photothermal-Responsive Shape-Changing Performance of the MPH Layer
2.4. NIR-Responsive Shape-Changing Performance of the Bi-Layer Hydrogel
2.5. The Bi-Functional Synergy Process of the Bi-Layer Hydrogel in the Dark
2.6. Biomimetic Devices Based on the Bi-Layer Hydrogel with Bi-Functional Synergy
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of pH-Responsive RhB
4.3. Bi-Layer Actuator Preparation
4.4. Characterization of Actuator
4.5. Swelling Ratio Test of Hydrogel
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Zhang, Q.; Li, X.; Serpe, M.J. Stimuli-responsive polymers for sensing and actuation. Mater. Horiz. 2019, 6, 1774–1793. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A Mussel-Inspired Polydopamine-Filled Cellulose Aerogel for Solar-Enabled Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Luo, H.; Han, J.; Chen, Y.; Jiang, J. Assessing the effects of cellulose-inorganic nanofillers on thermo/pH-dual responsive hydrogels. Appl. Surf. Sci. 2020, 528, 146961. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Jiang, S.; He, S.; Wu, Q.; Xiao, H.; Han, J. Environment-tolerant ionic hydrogel–elastomer hybrids with robust interfaces, high transparence, and biocompatibility for a mechanical–thermal multimode sensor. InfoMat 2023, 5, e12409. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Duan, G.; Fang, H.; Hou, H. Dense and thin coating of gel polymer electrolyte on sulfur cathode toward high performance Li-sulfur battery. Compos. Commun. 2020, 19, 239–245. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; McGonigal, P.R.; Ye, R.; Liu, S.; Lam, J.W.Y.; Kwok, R.T.K.; Yuan, W.Z.; Xie, J.; Rogach, A.L.; et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, D.; Jia, T.; Zhang, Y.; Meng, L.; Jin, Q.; Ma, H.; Lu, D.; Lai, S.; Lei, Z. Unprecedented Strong Photoluminescences Induced from Both Aggregation and Polymerization of Novel Nonconjugated β-Cyclodextrin Dimer. Ind. Eng. Chem. Res. 2017, 56, 3913–3919. [Google Scholar] [CrossRef]
- Xu, H.X.; Tan, Y.; Wang, D.; Wang, X.L.; An, W.L.; Xu, P.P.; Xu, S.; Wang, Y.Z. Autofluorescence of hydrogels without a fluorophore. Soft Matter 2019, 15, 3588–3594. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Zhu, C.N.; Bai, T.; Wang, H.; Bai, W.; Ling, J.; Sun, J.Z.; Huang, F.; Wu, Z.L.; Zheng, Q. Single Chromophore-Based White-Light-Emitting Hydrogel with Tunable Fluorescence and Patternability. ACS Appl. Mater. Interfaces 2018, 10, 39343–39352. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.; Wang, H.; Sun, P.; Li, T.; Lu, S.; Zhao, Y.; Wang, B. Smart Responsive Luminescent Aptamer-Functionalized Covalent Organic Framework Hydrogel for High-Resolution Visualization and Security Protection of Latent Fingerprints. ACS Appl. Mater. Interfaces 2019, 11, 44664–44672. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Han, S.W.; Park, C.; Lee, S.W.; Eoh, H.; Baek, J.; Shin, D.-G.; Park, T.H.; Huh, J.; Lee, H.; et al. 3D touchless multiorder reflection structural color sensing display. Sci. Adv. 2020, 6, eabb5769. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wei, S.; Liu, H.; Zhan, B.; Yan, H.; Lu, W.; Zhang, J.; Wu, S.; Chen, T. Programming Multistate Aggregation-Induced Emissive Polymeric Hydrogel into 3D Structures for On-Demand Information Decryption and Transmission. Adv. Intell. Syst. 2021, 3, 2000239. [Google Scholar] [CrossRef]
- Cayuela, A.; Kennedy, S.R.; Soriano, M.L.; Jones, C.D.; Valcarcel, M.; Steed, J.W. Fluorescent carbon dot-molecular salt hydrogels. Chem. Sci. 2015, 6, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sarkar, R.; Nandi, S.; Porgador, A.; Jelinek, R. Detection of Reactive Oxygen Species by a Carbon-Dot-Ascorbic Acid Hydrogel. Anal. Chem. 2017, 89, 830–836. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Wei, L.; Zhu, X.; Zhu, Y.; Wang, G.; Mei, T.; Li, J.; Wang, X. Construction of high-strength p(HEMA-co-AA) fluorescent hydrogels based on modified carbon dots as chemically crosslinkers. Colloid. Polym. Sci. 2018, 296, 745–752. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Guan, Y.; Zhang, Y. Polymerized microgel colloidal crystals: Photonic hydrogels with tunable band gaps and fast response rates. Angew Chem. Int. Ed. Engl. 2013, 52, 9961–9965. [Google Scholar] [CrossRef]
- Rafique, B.; Ullah Khan, R.; Sarfraz Rizvi, A.; Irfan, M.; Murtaza, G.; Qiu, L.; Xue, M.; Meng, Z. Creatinine Imprinted Photonic Crystals Hydrogel Sensor. Arab. J. Chem. 2023, 16, 104684. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Wang, J.; Wang, X.; Mbola, N.M.; Meng, Z.; Xue, M. Double-Network Hydrogel-Based Photonic Crystal Sensor for Mechanical Force Naked Eye Sensing and Its Application in Medical Compressive or Stretchy Instruments. ACS Appl. Mater. Interfaces 2023, 15, 2192–2203. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wan, J.; Jiang, S.; Wu, Y.; Wang, W.; Gong, X.; Wang, H. Quantum dots-based hydrogels for sensing applications. Chem. Eng. J. 2021, 408, 127351. [Google Scholar] [CrossRef]
- Deng, S.; Long, J.; Dai, X.; Wang, G.; Zhou, L. Simultaneous Detection and Adsorptive Removal of Cr(VI) Ions by Fluorescent Sulfur Quantum Dots Embedded in Chitosan Hydrogels. ACS Appl. Nano Mater. 2023, 6, 1817–1827. [Google Scholar] [CrossRef]
- Yan, J.; Pan, G.; Lin, W.; Tang, Z.; Zhang, J.; Li, J.; Li, W.; Lin, X.; Luo, H.; Yi, G. Multi-responsive graphene quantum dots hybrid self-healing structural color hydrogel for information encoding and encryption. Chem. Eng. J. 2023, 451, 138922. [Google Scholar] [CrossRef]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Q.; Wen, H.; Jiang, J.; Liu, L.; Duan, J. Preparation and performance of galactomannan temperature-sensitive hydrogels. J. For. Eng. 2021, 6, 120–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Lo, C.-Y.; Ruan, L.; Pi, C.-H.; Kim, C.; Alsaid, Y.; Frenkel, I.; Rico, R.; Tsao, T.-C.; He, X. Somatosensory actuator based on stretchable conductive photothermally responsive hydrogel. Sci. Robot. 2021, 6, eabd5483. [Google Scholar] [CrossRef]
- He, L.; Pan, L.; Zhou, W.; Niu, Z.; Chen, X.; Chen, M.; Zhang, Q.; Pan, W.; Xiao, P.; Li, Y. Thermal corrosion behavior of Yb4Hf3O12 ceramics exposed to calcium-ferrum-alumina-silicate (CFAS) at 1400 °C. J. Eur. Ceram. Soc. 2023, 43, 4114–4123. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Zhao, Y.; Hong, Y.; Tang, Y.; Yin, J. Bistable and Multistable Actuators for Soft Robots: Structures, Materials, and Functionalities. Adv. Mater. 2022, 34, e2110384. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Chen, M.; Dong, Y.; Zhou, X. Progress in preparation and application of anisotropic cellulose-based hydrogels. J. For. Eng. 2022, 7, 20–30. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, X.; Han, X.; Wang, P.; Zheng, W.J. Soft Actuator Based on Metal/Hydrogel Nanocomposites with Anisotropic Structure. Macromol. Chem. Phys. 2021, 223, 2100117. [Google Scholar] [CrossRef]
- Pan, L.; He, L.; Niu, Z.; Xiao, P.; Zhou, W.; Li, Y. Corrosion behavior of ytterbium hafnate exposed to water-vapor with Al(OH)3 impurities. J. Eur. Ceram. Soc. 2023, 43, 612–620. [Google Scholar] [CrossRef]
- Li, S.; Miao, Y.; Zhang, M.; Reheman, A.; Huang, L.; Chen, L.; Wu, H. Anti-UV antibacterial conductive lignin-copolymerized hydrogel for pressure sensing. J. For. Eng. 2022, 7, 114–123. [Google Scholar] [CrossRef]
- Cho, K.; Kang, D.; Lee, H.; Koh, W.-G. Multi-stimuli responsive and reversible soft actuator engineered by layered fibrous matrix and hydrogel micropatterns. Chem. Eng. J. 2022, 427, 130879. [Google Scholar] [CrossRef]
- Wang, T.; Huang, J.; Yang, Y.; Zhang, E.; Sun, W.; Tong, Z. Bioinspired Smart Actuator Based on Graphene Oxide-Polymer Hybrid Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 23423–23430. [Google Scholar] [CrossRef]
- Ko, J.; Kim, D.; Song, Y.; Lee, S.; Kwon, M.; Han, S.; Kang, D.; Kim, Y.; Huh, J.; Koh, J.S.; et al. Electroosmosis-Driven Hydrogel Actuators Using Hydrophobic/Hydrophilic Layer-By-Layer Assembly-Induced Crack Electrodes. ACS Nano 2020, 14, 11906–11918. [Google Scholar] [CrossRef]
- Liu, Y.; Takafuji, M.; Ihara, H.; Zhu, M.; Yang, M.; Gu, K.; Guo, W. Programmable responsive shaping behavior induced by visible multi-dimensional gradients of magnetic nanoparticles. Soft Matter 2012, 8, 3295–3299. [Google Scholar] [CrossRef]
- Ye, S.; Ma, W.; Fu, G. Anisotropic Hydrogels Constructed via a Novel Bilayer-Co-Gradient Structure Strategy toward Programmable Shape Deformation. Chem. Mater. 2023, 35, 999–1007. [Google Scholar] [CrossRef]
- Bai, H.; Polini, A.; Delattre, B.; Tomsia, A.P. Thermoresponsive composite hydrogels with aligned macroporous structure by ice-templated assembly. Chem. Mater. 2013, 25, 4551–4556. [Google Scholar] [CrossRef]
- Jiang, Z.; Seraji, S.M.; Tan, X.; Zhang, X.; Dinh, T.; Mollazade, M.; Rowan, A.E.; Whittaker, A.K.; Song, P.; Wang, H. Strong, Ultrafast, Reprogrammable Hydrogel Actuators with Muscle-Mimetic Aligned Fibrous Structures. Chem. Mater. 2021, 33, 7818–7828. [Google Scholar] [CrossRef]
- Wei, X.; Chen, L.; Wang, Y.; Sun, Y.; Ma, C.; Yang, X.; Jiang, S.; Duan, G. An Electrospinning Anisotropic Hydrogel with Remotely-Controlled Photo-Responsive Deformation and Long-Range Navigation for Synergist Actuation. Chem. Eng. J. 2022, 433, 134258. [Google Scholar] [CrossRef]
- Kim, J.; Hanna, J.A.; Byun, M.; Santangelo, C.D.; Hayward, R.C. Designing responsive buckled surfaces by halftone gel lithography. Science 2012, 335, 1201–1205. [Google Scholar] [CrossRef]
- Wu, Z.L.; Moshe, M.; Greener, J.; Therien-Aubin, H.; Nie, Z.; Sharon, E.; Kumacheva, E. Three-dimensional shape transformations of hydrogel sheets induced by small-scale modulation of internal stresses. Nat. Commun. 2013, 4, 1586. [Google Scholar] [CrossRef]
- Peng, X.; Liu, T.; Zhang, Q.; Shang, C.; Bai, Q.-W.; Wang, H. Surface Patterning of Hydrogels for Programmable and Complex Shape Deformations by Ion Inkjet Printing. Adv. Funct. Mater. 2017, 27, 1701962. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Du, C.; Dai, Y.; Daab, M.; Matejdes, M.; Breu, J.; Hong, W.; Zheng, Q.; Wu, Z.L. Light-steered locomotion of muscle-like hydrogel by self-coordinated shape change and friction modulation. Nat. Commun. 2020, 11, 5166. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gevorkian, A.; Morozova, S.M.; Kheiri, S.; Khuu, N.; Chen, H.; Young, E.; Yan, N.; Kumacheva, E. Actuation of Three-Dimensional-Printed Nanocolloidal Hydrogel with Structural Anisotropy. Adv. Funct. Mater. 2021, 31, 2010743. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; Lv, F.; Liu, L.; Gu, Q.; Wang, S. Biomimetic 4D-Printed Breathing Hydrogel Actuators by Nanothylakoid and Thermoresponsive Polymer Networks. Adv. Funct. Mater. 2021, 31, 2105544. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, Y.; Wang, X.; An, W.; Xu, S.; Liao, W.; Wang, Y. Photothermal Nanocomposite Hydrogel Actuator with Electric-Field-Induced Gradient and Oriented Structure. ACS Appl. Mater. Interfaces 2018, 10, 7688–7692. [Google Scholar] [CrossRef]
- Ma, C.; Lu, W.; Yang, X.; He, J.; Le, X.; Wang, L.; Zhang, J.; Serpe, M.J.; Huang, Y.; Chen, T. Bioinspired Anisotropic Hydrogel Actuators with On-Off Switchable and Color-Tunable Fluorescence Behaviors. Adv. Funct. Mater. 2018, 28, 1704568. [Google Scholar] [CrossRef]
- Wei, S.; Lu, W.; Le, X.; Ma, C.; Lin, H.; Wu, B.; Zhang, J.; Theato, P.; Chen, T. Bioinspired Synergistic Fluorescence-Color-Switchable Polymeric Hydrogel Actuators. Angew. Chem. Int. Ed. Engl. 2019, 58, 16243–16251. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Ji, X.; Gong, J.; Hu, Y.; Wu, W.; Wang, X.; Peng, H.Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Bioinspired Simultaneous Changes in Fluorescence Color, Brightness, and Shape of Hydrogels Enabled by AIEgens. Adv. Mater. 2020, 32, e1906493. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Y.; Lu, W.; Wu, B.; Wei, S.; Wu, S.; Wang, W.; Chen, T. Bio-inspired Structure-editing Fluorescent Hydrogel Actuators for Environment-interactive Information Encryption. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300417. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Peng, S.; Chen, L.; Cao, X.; Sun, Y.; Chen, L.; Yang, L.; Ma, C.; Liu, Q.; Liu, Z.; et al. Anisotropic Bi-Layer Hydrogel Actuator with pH-Responsive Color-Changing and Photothermal-Responsive Shape-Changing Bi-Functional Synergy. Gels 2023, 9, 438. https://doi.org/10.3390/gels9060438
Ma C, Peng S, Chen L, Cao X, Sun Y, Chen L, Yang L, Ma C, Liu Q, Liu Z, et al. Anisotropic Bi-Layer Hydrogel Actuator with pH-Responsive Color-Changing and Photothermal-Responsive Shape-Changing Bi-Functional Synergy. Gels. 2023; 9(6):438. https://doi.org/10.3390/gels9060438
Chicago/Turabian StyleMa, Chao, Shuyi Peng, Lian Chen, Xingyu Cao, Ye Sun, Lin Chen, Lang Yang, Chunming Ma, Qijie Liu, Zhenzhong Liu, and et al. 2023. "Anisotropic Bi-Layer Hydrogel Actuator with pH-Responsive Color-Changing and Photothermal-Responsive Shape-Changing Bi-Functional Synergy" Gels 9, no. 6: 438. https://doi.org/10.3390/gels9060438