Superhydrophobic/Superoleophilic PDMS/SiO2 Aerogel Fabric Gathering Device for Self-Driven Collection of Floating Viscous Oil
Abstract
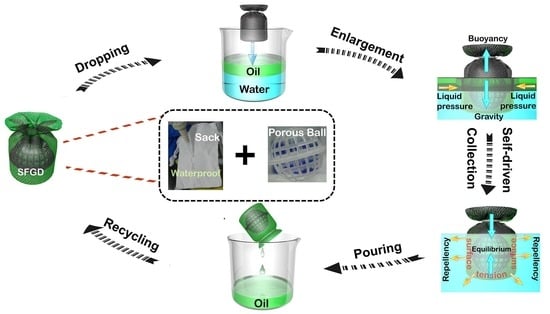
:1. Introduction
2. Results and Discussion
2.1. Mechanism and Surface Wettability
2.2. Surface Chemical Component Analysis
2.3. Mechanical Robustness
2.4. Separation of Viscous Oil/Water Mixture
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Superhydrophobic and Superoleophilic Burlap Sack
4.3. Characterization
4.4. Stability Test
4.5. Separation of the Immiscible Viscous Oil/Water Mixture
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Ye, Y.; Li, Z.; Chen, X.; Bai, Q.; Wang, K.; Zhang, Y.; Drioli, E.; Ma, J. Constructing Environmental-Friendly “Oil-Diode” Janus Membrane for Oil/Water Separation. ACS Nano 2022, 16, 4684–4692. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhu, Y.; Fang, W.; Ji, M.; Wang, A.; Gao, S.; Lin, H.; Huang, R.; Jin, J. Double-Defense Design of Super-Anti-Fouling Membranes for Oil/Water Emulsion Separation. Adv. Funct. Mater. 2022, 32, 2113247. [Google Scholar] [CrossRef]
- Song, J.; Huang, S.; Lu, Y.; Bu, X.; Mates, J.E.; Ghosh, A.; Ganguly, R.; Carmalt, C.J.; Parkin, I.P.; Xu, W.; et al. Self-driven one-step oil removal from oil spill on water via selective-wettability steel mesh. ACS Appl. Mater. Interfaces 2014, 6, 19858–19865. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, L.; Liu, J.; He, S.; Shao, W. Sustainable, Highly Efficient and Superhydrophobic Fluorinated Silica Functionalized Chitosan Aerogel for Gravity-Driven Oil/Water Separation. Gels 2021, 7, 66. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Li, Y.; Zhang, W.; Bai, S.; Zheng, X.; Huan, J.; Cao, G.; Yang, T.; Wang, M.; et al. Development of a facile and bi-functional superhydrophobic suspension and its applications in superhydrophobic coatings and aerogels in high-efficiency oil–water separation. Green Chem. 2020, 22, 7424–7434. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.-N.; Chen, Z.-X.; He, A.; Zhong, Q.-Z.; Xu, Z.-K. Janus membranes with controllable asymmetric configurations for highly efficient separation of oil-in-water emulsions. J. Mater. Chem. A 2019, 7, 7907–7917. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Chen, G.; Pei, Y.; Pastel, G.; Jia, C.; Song, J.; Mi, R.; Yang, B.; Das, S.; et al. Bioinspired Solar-Heated Carbon Absorbent for Efficient Cleanup of Highly Viscous Crude Oil. Adv. Funct. Mater. 2019, 29, 1900162. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. [Google Scholar] [CrossRef]
- Remuiñán-Pose, P.; López-Iglesias, C.; Iglesias-Mejuto, A.; Mano, J.F.; García-González, C.A.; Rial-Hermida, M.I. Preparation of Vancomycin-Loaded Aerogels Implementing Inkjet Printing and Superhydrophobic Surfaces. Gels 2022, 8, 417. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Luo, S.; Cheng, C.; Wang, S.; Liu, B. Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation. Coatings 2022, 12, 1698. [Google Scholar] [CrossRef]
- Peng, K.; Wang, C.; Chang, C.; Peng, N. Phosphonium Modified Nanocellulose Membranes with High Permeate Flux and Antibacterial Property for Oily Wastewater Separation. Coatings 2022, 12, 1598. [Google Scholar] [CrossRef]
- Sperling, M.; Gradzielski, M. Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials. Gels 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Sun, J.; Ma, C.; Luo, S.; Li, W.; Liu, S. pH-Responsive Carbon Foams with Switchable Wettability Made from Larch Sawdust for Oil Recovery. Polymers 2023, 15, 638. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Xu, X.; Mredha, M.T.I.; Cui, J.; Vlassak, J.J.; Jeon, I. Hydrogel bowls for cleaning oil spills on water. Water Res. 2018, 145, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, J.S.; Lee, G.; Seo, D.K.; Baek, Y.; Yoon, J.; Oh, S.M.; Kang, T.J.; Lee, H.H.; Kim, Y.H. Autonomous Graphene Vessel for Suctioning and Storing Liquid Body of Spilled Oil. Sci. Rep. 2016, 6, 22339. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Yang, H.C.; Xie, Y.; Chan, H.; Narayanan, B.; Chen, L.; Waldman, R.Z.; Sankaranarayanan, S.; Elam, J.W.; Darling, S.B. Crude-Oil-Repellent Membranes by Atomic Layer Deposition: Oxide Interface Engineering. ACS Nano 2018, 12, 8678–8685. [Google Scholar] [CrossRef]
- Wu, M.-B.; Hong, Y.-M.; Liu, C.; Yang, J.; Wang, X.-P.; Agarwal, S.; Greiner, A.; Xu, Z.-K. Delignified wood with unprecedented anti-oil properties for the highly efficient separation of crude oil/water mixtures. J. Mater. Chem. A 2019, 7, 16735–16741. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Fei, B.; Hu, H.; Lai, C.; Xin, J.H. Bioinspired, Stimuli-Responsive, Multifunctional Superhydrophobic Surface with Directional Wetting, Adhesion, and Transport of Water. Adv. Funct. Mater. 2015, 25, 5047–5056. [Google Scholar] [CrossRef]
- Nam, C.; Li, H.; Zhang, G.; Lutz, L.R.; Nazari, B.; Colby, R.H.; Chung, T.C.M. Practical Oil Spill Recovery by a Combination of Polyolefin Absorbent and Mechanical Skimmer. ACS Sustain. Chem. Eng. 2018, 6, 12036–12045. [Google Scholar] [CrossRef]
- Dong, C.; Hu, Y.; Zhu, Y.; Wang, J.; Jia, X.; Chen, J.; Li, J. Fabrication of Textile Waste Fibers Aerogels with Excellent Oil/Organic Solvent Adsorption and Thermal Properties. Gels 2022, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, Y.; Feng, J.; Li, L.; Feng, J. Bionic Aerogel with a Lotus Leaf-like Structure for Efficient Oil-Water Separation and Electromagnetic Interference Shielding. Gels 2023, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Bayraktaroglu, S.; Kizil, S.; Bulbul Sonmez, H. A highly reusable polydimethylsiloxane sorbents for oil/organic solvent clean-up from water. J. Environ. Chem. Eng. 2021, 9, 106002. [Google Scholar] [CrossRef]
- Pandey, K.; Bindra, H.S.; Jain, S.; Nayak, R. Sustainable lotus leaf wax nanocuticles integrated polydimethylsiloxane sorbent for instant removal of oily waste from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127937. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, X.; Chao, Y.; Chen, D.; Liao, Y. Super-Hydrophobic Magnetic Fly Ash Coated Polydimethylsiloxane (MFA@PDMS) Sponge as an Absorbent for Rapid and Efficient Oil/Water Separation. Polymers 2022, 14, 3726. [Google Scholar] [CrossRef]
- Qiu, S.; Bi, H.; Hu, X.; Wu, M.; Li, Y.; Sun, L. Moldable clay-like unit for synthesis of highly elastic polydimethylsiloxane sponge with nanofiller modification. RSC Adv. 2017, 7, 10479–10486. [Google Scholar] [CrossRef]
- Mo, S.; Mei, J.; Liang, Q.; Li, Z. Repeatable oil-water separation with a highly-elastic and tough amino-terminated polydimethylsiloxane-based sponge synthesized using a self-foaming method. Chemosphere 2021, 271, 129827. [Google Scholar] [CrossRef]
- Prasanthi, I.; Raidongia, K.; Datta, K.K.R. Super-wetting properties of functionalized fluorinated graphene and its application in oil–water and emulsion separation. Mater. Chem. Front. 2021, 5, 6244–6255. [Google Scholar] [CrossRef]
- Cao, C.; Ge, M.; Huang, J.; Li, S.; Deng, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust fluorine-free superhydrophobic PDMS–ormosil@fabrics for highly effective self-cleaning and efficient oil–water separation. J. Mater. Chem. A 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Xu, L.; Wan, J.; Yuan, X.; Pan, H.; Wang, L.; Shen, Y.; Sheng, Y. Preparation of durable superamphiphobic cotton fabrics with self-cleaning and liquid repellency. J. Adhes. Sci. Technol. 2022, 36, 1–20. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Liang, T.; Feng, Y.; Zeng, X. Polydimethylsiloxane-Based Superhydrophobic Surfaces on Steel Substrate: Fabrication, Reversibly Extreme Wettability and Oil-Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 3131–3141. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Sun, Q.; Zhong, Q.; Mu, P.; Li, J. Current research situation and future prospect of superwetting smart oil/water separation materials. J. Mater. Chem. A 2022, 10, 20190–20217. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation Mechanism and Construction of Surfaces with Special Wettability for Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, G.; Zhang, Q.; Zhan, X.; Chen, F. Silicone Oil-Infused Slippery Surfaces Based on Sol-Gel Process-Induced Nanocomposite Coatings: A Facile Approach to Highly Stable Bioinspired Surface for Biofouling Resistance. ACS Appl. Mater. Interfaces 2016, 8, 34810–34819. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zeng, X.; Li, H.; Lai, X. Facile fabrication of a robust superhydrophobic/superoleophilic sponge for selective oil absorption from oily water. RSC Adv. 2014, 4, 23861. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Di, X.; Sun, X.; Wang, X.; Yang, T.; Wang, M.; Li, J.; Wang, C.; Li, Y. Superhydrophobic/Superoleophilic PDMS/SiO2 Aerogel Fabric Gathering Device for Self-Driven Collection of Floating Viscous Oil. Gels 2023, 9, 405. https://doi.org/10.3390/gels9050405
Liu F, Di X, Sun X, Wang X, Yang T, Wang M, Li J, Wang C, Li Y. Superhydrophobic/Superoleophilic PDMS/SiO2 Aerogel Fabric Gathering Device for Self-Driven Collection of Floating Viscous Oil. Gels. 2023; 9(5):405. https://doi.org/10.3390/gels9050405
Chicago/Turabian StyleLiu, Feng, Xin Di, Xiaohan Sun, Xin Wang, Tinghan Yang, Meng Wang, Jian Li, Chengyu Wang, and Yudong Li. 2023. "Superhydrophobic/Superoleophilic PDMS/SiO2 Aerogel Fabric Gathering Device for Self-Driven Collection of Floating Viscous Oil" Gels 9, no. 5: 405. https://doi.org/10.3390/gels9050405