Biomass Chitosan-Based Tubular/Sheet Superhydrophobic Aerogels Enable Efficient Oil/Water Separation
Abstract
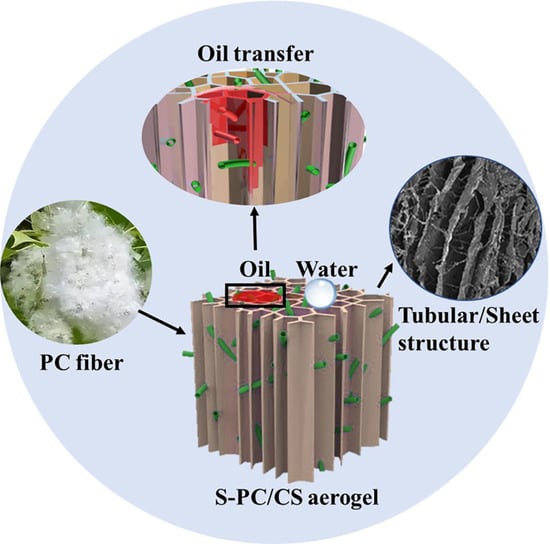
:1. Introduction
2. Results and Discussion
| Compressive Properties | |||||||
|---|---|---|---|---|---|---|---|
| Sorbent Material | Maximum Compression Stress (kPa) | Number of Compression Cycles | Plastic Deformation | WCA | Sorption Capacity (g/g−1) | Preparation Method | Reference |
| Lignin/Agarose/PVA aerogels | <16 | 10 | 20% | 150º | 18 | Indirectional freeze-drying | Jiang, J., et al., 2017 [43] |
| Dialdehyde carboxymethyl cellulose aerogels | <10 | 50 | 15–20% | 144.5º | 20–30 | Indirectional freeze-drying | Zhang, F., et al., 2022 [35] |
| Cellulose nanocrystals/PVA aerogels | <35 | 50 | >15% | 136º | <35 | Indirectional freeze-drying | Gong, X., et al., 2019 [36] |
| Carboxylated cellulose nanofibers/PEI aerogels | <9 | 1 | 20% | - | 20–60 | Indirectional freeze-drying | Tang, R., et al., 2023 [37] |
| Cellulose nanofibrils/N-alkylated chitosan/poly(vinyl alcohol) aerogels | <55 kPa | 50 | 18–20% | 147º | 19–51 | Indirectional freeze-drying | Li, M., et al., 2021 [44] |
| Seed hairs of typha orientalis aerogels | <25 | 10 | 14.8% | 153º | 42–160 | Carbonized | Yang, J., et al., 2018 [38] |
| Bacterial cellulose aerogels | <3.5 | 100 | 5% | 131 ± 3.5º | 37–89 | Pyrolysis | Ieamviteevanich, P., et al., 2020 [45] |
| Alginate/oil gelator aerogels | <9 kPa | - | - | 155 ± 5º | 32 | Indirectional freeze-drying | Wang, Y., et al., 2022 [42] |
| graphene oxide/halloysite nanotubes (RGO/HNTs) membrane | - | - | 82.43º | - | Hummers method | Liu Y., et al., 2018 [39] | |
| chitin/halloysite nanotubes sponge | - | - | - | 88–98º | 11.23 | Freeze-drying | Zhao X, et al., 2019 [40] |
| HNTC-FG-PU sponges | - | - | - | 145 ± 2º | 50.8 | Dip-coating | Prasanthi, I., et al., 2022 [41] |
| S-PC/CS aerogels | 16.5 (ε = 60%) | 50 | 8.25% | 154 ± 0.4º | 33.06–73.22 | YES | This work |
3. Conclusions
4. Material and Methods
4.1. Materials
4.2. Pre-Treatment of PC Fibers
4.3. Preparation of Aerogels
4.4. Modification of Aerogels
4.5. Characterizations
4.6. Oil Sorption Capacity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hua, Y.; Dong, T. Multi-functional flame-retardant superhydrophobic ceramic fiber felt: Oil/Water mixture separation and oil mist interception. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127454. [Google Scholar] [CrossRef]
- Dong, T.; Li, Q.; Tian, N.; Zhao, H.; Zhang, Y.; Han, G. Concus Finn Capillary driven fast viscous oil-spills removal by superhydrophobic cruciate polyester fibers. J. Hazard. Mater. 2021, 417, 126133. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Liu, Y.; Tian, N.; Zhang, Y.; Han, G.; Peng, F.; Lou, C.-W.; Chi, S.; Liu, Y.; Liu, C.; et al. Photothermal and Concus Finn capillary assisted superhydrophobic fibrous network enabling instant viscous oil transport for crude oil cleanup. J. Hazard. Mater. 2023, 443, 130193. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Geng, R.; Lv, X. Are oil spills enhancing outbreaks of red tides in the Chinese coastal waters from 1973 to 2017? Environ. Sci. Pollut. Res. 2021, 28, 56473–56479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, T.; Cui, W.; Wang, X.; Ramakrishna, S.; Long, Y. Harsh environment-tolerant and robust PTFE@ZIF-8 fibrous membrane for efficient photocatalytic organic pollutants degradation and oil/water separation. Sep. Purif. Technol. 2023, 306, 122586. [Google Scholar] [CrossRef]
- Li, T.-T.; Li, S.; Sun, F.; Shiu, B.-C.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. pH-responsive nonwoven fabric with reversibly wettability for controllable oil-water separation and heavy metal removal. Environ. Res. 2022, 215, 114355. [Google Scholar] [CrossRef]
- Li, K.; Yu, H.; Yan, J.; Liao, J. Analysis of Offshore Oil Spill Pollution Treatment Technology. IOP Conf. Ser. Earth Environ. Sci. 2020, 510, 042011. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Cherukupally, P.; Bilton, A.M.; Park, C.B. Novel separator skimmer for oil spill cleanup and oily wastewater treatment: From conceptual system design to the first pilot-scale prototype development. Environ. Technol. Innov. 2020, 18, 100598. [Google Scholar] [CrossRef]
- Li, Z.-T.; Wu, H.-T.; Chen, W.-Y.; He, F.-A.; Li, D.-H. Preparation of magnetic superhydrophobic melamine sponges for effective oil-water separation. Sep. Purif. Technol. 2019, 212, 40–50. [Google Scholar] [CrossRef]
- Wu, F.; Pickett, K.; Panchal, A.; Liu, M.; Lvov, Y. Superhydrophobic Polyurethane Foam Coated with Polysiloxane-Modified Clay Nanotubes for Efficient and Recyclable Oil Absorption. ACS Appl. Mater. Interfaces 2019, 11, 25445–25456. [Google Scholar] [CrossRef]
- Jin, X.; Al-Qatatsheh, A.; Subhani, K.; Salim, N.V. Biomimetic and flexible 3D carbon nanofiber networks with fire-resistant and high oil-sorption capabilities. Chem. Eng. J. 2021, 412, 128635. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Pan, Z.; Guan, Y.; Liu, Y.; Cheng, F. Facile fabrication of hydrophobic and underwater superoleophilic elastic and mechanical robust graphene/PDMS sponge for oil/water separation. Sep. Purif. Technol. 2021, 261, 118273. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, W.; Song, Y.; Zheng, Q. Graphene/nanofiber aerogels: Performance regulation towards multiple applications in dye adsorption and oil/water separation. Chem. Eng. J. 2018, 338, 202–210. [Google Scholar] [CrossRef]
- Dong, T.; Cao, S.; Xu, G. Highly efficient and recyclable depth filtrating system using structured kapok filters for oil removal and recovery from wastewater. J. Hazard. Mater. 2017, 321, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Cao, S.; Xu, G. Highly porous oil sorbent based on hollow fibers as the interceptor for oil on static and running water. J. Hazard. Mater. 2016, 305, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, C.; Liu, S.; Zhang, Y.; Kong, X.; Wang, M.; Ding, C.; Liu, T.; Shen, H.; Bi, H. Super-hydrophobic cotton aerogel with ultra-high flux and high oil retention capability for efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130572. [Google Scholar] [CrossRef]
- Dong, T.; Tian, N.; Xu, B.; Huang, X.; Chi, S.; Liu, Y.; Lou, C.-W.; Lin, J.-H. Biomass poplar catkin fiber-based superhydrophobic aerogel with tubular-lamellar interweaved neurons-like structure. J. Hazard. Mater. 2022, 429, 128290. [Google Scholar] [CrossRef]
- Panahi, S.; Moghaddam, M.K.; Moezzi, M. Assessment of milkweed floss as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. J. Environ. Manag. 2020, 268, 110688. [Google Scholar] [CrossRef]
- Tian, N.; Wu, S.; Han, G.; Zhang, Y.; Li, Q.; Dong, T. Biomass-derived oriented neurovascular network-like superhydrophobic aerogel as robust and recyclable oil droplets captor for versatile oil/water separation. J. Hazard. Mater. 2022, 424, 127393. [Google Scholar] [CrossRef]
- Dong, T.; Li, Q.; Nie, K.; Jiang, W.; Li, S.; Hu, X.; Han, G. Facile Fabrication of Marine Algae-Based Robust Superhydrophobic Sponges for Efficient Oil Removal from Water. ACS Omega 2020, 5, 21745–21752. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, Y.; Feng, J.; Li, L.; Feng, J. Bionic Aerogel with a Lotus Leaf-like Structure for Efficient Oil-Water Separation and Electromagnetic Interference Shielding. Gels 2023, 9, 214. [Google Scholar] [CrossRef]
- Ma, X.; Tian, N.; Wang, G.; Wang, W.; Miao, J.; Fan, T. Biomimetic vertically aligned aerogel with synergistic photothermal effect enables efficient solar-driven desalination. Desalination 2023, 550, 116397. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ke, W.-T.; Liao, Y.-C. Elastic nanocellulose/graphene aerogel with excellent shape retention and oil absorption selectivity. J. Taiwan Inst. Chem. Eng. 2020, 111, 261–269. [Google Scholar] [CrossRef]
- Hu, X.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Ramakrishna, S.; Wang, X.; Yao, J. Preparation of high elastic bacterial cellulose aerogel through thermochemical vapor deposition catalyzed by solid acid for oil-water separation. Carbohydr. Polym. 2023, 305, 120538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z. Anisotropic Cellulose Nanofibers/Polyvinyl Alcohol/Graphene Aerogels Fabricated by Directional Freeze-drying as Effective Oil Adsorbents. Polymers 2019, 11, 712. [Google Scholar] [CrossRef]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from Water. J. Hazard. Mater. 2020, 385, 121507. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Cheng, M.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Shen, M.; Song, B.; Liu, J.; Yang, H.; et al. Insights into catalytic removal and separation of attached metals from natural-aged microplastics by magnetic biochar activating oxidation process. Water Res. 2020, 179, 115876. [Google Scholar] [CrossRef]
- He, S.; Jia, M.; Xiang, Y.; Song, B.; Xiong, W.; Cao, J.; Peng, H.; Yang, Y.; Wang, W.; Yang, Z.; et al. Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. J. Hazard. Mater. 2022, 424, 127286. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Khandelwal, N.; Tiwari, E.; Singh, N.; Darbha, G.K. Biochar-facilitated remediation of nanoplastic contaminated water: Effect of pyrolysis temperature induced surface modifications. J. Hazard. Mater. 2021, 417, 126096. [Google Scholar] [CrossRef]
- Rocha-Pimienta, J.; Navajas-Preciado, B.; Barraso-Gil, C.; Martillanes, S.; Delgado-Adámez, J. Optimization of the Extraction of Chitosan and Fish Gelatin from Fishery Waste and Their Antimicrobial Potential as Active Biopolymers. Gels 2023, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Chijcheapaza-Flores, H.; Tabary, N.; Chai, F.; Maton, M.; Staelens, J.-N.; Cazaux, F.; Neut, C.; Martel, B.; Blanchemain, N.; Garcia-Fernandez, M.J. Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections. Gels 2023, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gu, J.; Tu, D.; Guan, L.; Hu, C. Efficient Hydrophobic Modification of Old Newspaper and Its Application in Paper Fiber Reinforced Composites. Polymers 2019, 11, 842. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Mu, C.; Lin, W. A novel hydrophobic all-biomass aerogel reinforced by dialdehyde carboxymethyl cellulose for oil/organic solvent-water separation. Polymer 2022, 238, 124402. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Zeng, H.; Betti, M.; Chen, L. Highly Porous, Hydrophobic, and Compressible Cellulose Nanocrystals/Poly(vinyl alcohol) Aerogels as Recyclable Absorbents for Oil–Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 11118–11128. [Google Scholar] [CrossRef]
- Tang, R.; Xu, S.; Hu, Y.; Wang, J.; Lu, C.; Wang, L.; Zhou, Z.; Liao, D.; Zhang, H.; Tong, Z. Multifunctional nano-cellulose aerogel for efficient oil–water separation: Vital roles of magnetic exfoliated bentonite and polyethyleneimine. Sep. Purif. Technol. 2023, 314, 123557. [Google Scholar] [CrossRef]
- Yang, J.; Xu, P.; Xia, Y.; Chen, B. Multifunctional carbon aerogels from typha orientalis for oil/water separation and simultaneous removal of oil-soluble pollutants. Cellulose 2018, 25, 5863–5875. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, W.; Chen, M.; Ma, L.; Yang, B.; Liang, Q.; Chen, Y. A mussel-induced method to fabricate reduced graphene oxide/halloysite nanotubes membranes for multifunctional applications in water purification and oil/water separation. Chem. Eng. J. 2018, 336, 263–277. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, Y.; Tan, P.; Liu, M.; Zhou, C. Hydrophobically modified chitin/halloysite nanotubes composite sponges for high efficiency oil-water separation. Int. J. Biol. Macromol. 2019, 132, 406–415. [Google Scholar] [CrossRef]
- Prasanthi, I.; Rani Bora, B.; Raidongia, K.; Datta, K.K.R. Fluorinated graphene nanosheet supported halloysite nanoarchitectonics: Super-wetting coatings for efficient and recyclable oil sorption. Sep. Purif. Technol. 2022, 301, 122049. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Fan, W.; Liu, R.; Liu, Y. Alginate-oil gelator composite foam for effective oil spill treatment. Carbohydr. Polym. 2022, 294, 119755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. Renewable, Biomass-Derived, Honeycomblike Aerogel As a Robust Oil Absorbent with Two-Way Reusability. ACS Sustain. Chem. Eng. 2017, 5, 10307–10316. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Liu, J.; Pei, Y.; Zheng, X.; Tang, K.; Wang, F. Hydrophobic and self-recoverable cellulose nanofibrils/N-alkylated chitosan/poly(vinyl alcohol) sponge for selective and versatile oil/water separation. Int. J. Biol. Macromol. 2021, 192, 169–179. [Google Scholar] [CrossRef]
- Ieamviteevanich, P.; Palaporn, D.; Chanlek, N.; Poo-arporn, Y.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Carbon Nanofiber Aerogel/Magnetic Core–Shell Nanoparticle Composites as Recyclable Oil Sorbents. ACS Appl. Nano Mater. 2020, 3, 3939–3950. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lin, J.-H.; Guo, J.; Sun, R.; Han, G.; Peng, F.; Chi, S.; Dong, T. Biomass Chitosan-Based Tubular/Sheet Superhydrophobic Aerogels Enable Efficient Oil/Water Separation. Gels 2023, 9, 346. https://doi.org/10.3390/gels9040346
Wang W, Lin J-H, Guo J, Sun R, Han G, Peng F, Chi S, Dong T. Biomass Chitosan-Based Tubular/Sheet Superhydrophobic Aerogels Enable Efficient Oil/Water Separation. Gels. 2023; 9(4):346. https://doi.org/10.3390/gels9040346
Chicago/Turabian StyleWang, Wenhui, Jia-Horng Lin, Jiali Guo, Rui Sun, Guangting Han, Fudi Peng, Shan Chi, and Ting Dong. 2023. "Biomass Chitosan-Based Tubular/Sheet Superhydrophobic Aerogels Enable Efficient Oil/Water Separation" Gels 9, no. 4: 346. https://doi.org/10.3390/gels9040346