Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury
Abstract
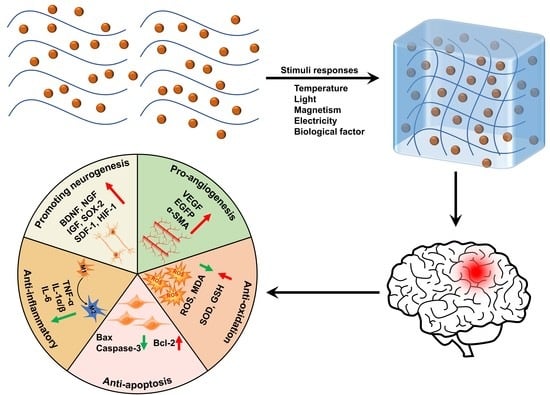
:1. Introduction
2. Stimuli-Responsive Hydrogels
2.1. Thermo-Responsive Hydrogels
2.2. Photoresponsive Hydrogels
2.3. Magnetic-Responsive Hydrogels
2.4. Electroresponsive Hydrogels
2.5. Bioresponsive Hydrogels
3. Applications of Stimuli-Responsive Hydrogels in TBI
4. Therapeutic Mechanisms of Stimuli-Responsive Hydrogels in TBI
4.1. Pro-Neurogenesis
4.2. Anti-Inflammation
4.3. Anti-Apoptosis
4.4. Anti-Oxidation
4.5. Pro-Angiogenesis
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haarbauer-Krupa, J.; Pugh, M.J.; Prager, E.M.; Harmon, N.; Wolfe, J.; Yaffe, K. Epidemiology of Chronic Effects of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3235–3247. [Google Scholar] [CrossRef]
- Jassam, Y.N.; Izzy, S.; Whalen, M.; McGavern, D.B.; El Khoury, J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 2017, 95, 1246–1265. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zheng, J.; Xu, S.; Fang, Y.; Wu, Y.; Zeng, J.; Shao, A.; Shi, L.; Lu, J.; Mei, S.; et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J. Neuroinflamm. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Eigenbrodt, A.K.; Seifert, T.; Sinclair, A.J.; Scher, A.I.; Schytz, H.W.; Lee, M.J.; De Icco, R.; Finkel, A.G.; Ashina, M. Post-traumatic headache attributed to traumatic brain injury: Classification, clinical characteristics, and treatment. Lancet Neurol. 2021, 20, 460–469. [Google Scholar] [CrossRef]
- Jiang, J.-Y.; Gao, G.-Y.; Feng, J.-F.; Mao, Q.; Chen, L.-G.; Yang, X.-F.; Liu, J.-F.; Wang, Y.-H.; Qiu, B.-H.; Huang, X.-J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Tan, H.X.; Borgo, M.P.D.; Aguilar, M.I.; Forsythe, J.S.; Taylor, J.M.; Crack, P.J. The use of bioactive matrices in regenerative therapies for traumatic brain injury. Acta Biomater. 2020, 102, 1–12. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Krishnakumar, G.S.; Giusto, E.; Furlani, F.; Bassi, G.; Rossi, A.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2022, 140, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio. 2022, 13, 100186. [Google Scholar]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Del. Rev. 2001, 53, 321–339. [Google Scholar]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2020, 31, 2005941. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 758–780. [Google Scholar] [CrossRef] [Green Version]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.J.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B.; et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar]
- Avila-Salas, F.; Duran-Lara, E.F. An Overview, of Injectable Thermo-Responsive Hydrogens and Advances in their Biomedical Applications. Curr. Med. Chem. 2020, 27, 5773–5789. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasinski, A.; Zielinska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Chee, P.L.; Young, D.J.; Loh, X.J. Chapter 7 Degradation Behaviour of Biodegradable Thermogels. In Biodegradable Thermogels; The Royal Society of Chemistry: London, UK, 2019; pp. 113–132. [Google Scholar]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef]
- Gupta, M.K.; Martin, J.R.; Werfel, T.A.; Shen, T.; Page, J.M.; Duvall, C.L. Cell protective, ABC triblock polymer-based thermoresponsive hydrogels with ROS-triggered degradation and drug release. J. Am. Chem. Soc. 2014, 136, 14896–14902. [Google Scholar] [CrossRef]
- Mahlumba, P.; Kumar, P.; du Toit, L.C.; Poka, M.S.; Ubanako, P.; Choonara, Y.E. Fabrication and Characterisation of a Photo-Responsive, Injectable Nanosystem for Sustained Delivery of Macromolecules. Int. J. Mol. Sci. 2021, 22, 3359. [Google Scholar]
- Ji, W.; Wu, Q.; Han, X.; Zhang, W.; Wei, W.; Chen, L.; Li, L.; Huang, W. Photosensitive hydrogels: From structure, mechanisms, design to bioapplications. Sci. China Life Sci. 2020, 63, 1813–1828. [Google Scholar]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, e1807333. [Google Scholar]
- Suzuki, A. Light-induced phase-transition of poly(n-isopropylacrylamide-co-chlorophyllin) gels. JIMSS 1994, 5, 112–116. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Design of Thiol- and Light-sensitive Degradable Hydrogels using Michael-type Addition Reactions. Polym. Chem. 2015, 6, 5565–5574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugao, A.B.; Malmonge, S.M. Use of radiation in the production of hydrogels. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2001, 185, 37–42. [Google Scholar] [CrossRef]
- Wang, H.; Jin, Y.; Tan, Y.; Zhu, H.; Huo, W.; Niu, P.; Li, Z.; Zhang, J.; Liang, X.J.; Yang, X. Photo-responsive hydrogel facilitates nutrition deprivation by an ambidextrous approach for preventing cancer recurrence and metastasis. Biomaterials 2021, 275, 120992. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Zhang, Q. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Q.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. 2016, 52, 978–981. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic Hydrogels and Their Potential Biomedical Applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Shi, W.; Huang, J.; Fang, R.; Liu, M. Imparting Functionality to the Hydrogel by Magnetic-Field-Induced Nano-assembly and Macro-response. ACS Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar]
- Zhang, J.; Huang, Q.; Du, J. Recent advances in magnetic hydrogels. Polym. Int. 2016, 65, 1365–1372. [Google Scholar] [CrossRef]
- Santhosh, M.; Choi, J.H.; Choi, J.W. Magnetic-Assisted Cell Alignment within a Magnetic Nanoparticle-Decorated Reduced Graphene Oxide/Collagen 3D Nanocomposite Hydrogel. Nanomaterials 2019, 9, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veloso, S.R.S.; Andrade, R.G.D.; Castanheira, E.M.S. Review on the advancements of magnetic gels: Towards multifunctional magnetic liposome-hydrogel composites for biomedical applications. Adv. Colloid Interface Sci. 2021, 288, 102351. [Google Scholar] [CrossRef] [PubMed]
- Ramanujan, R.V.; Lao, L.L. The mechanical behavior of smart magnet-hydrogel composites. SmMaS 2006, 15, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.Q.; Ahmed, A.S.; Ramanujan, R.V. Morphing soft magnetic composites. Adv. Mater. 2012, 24, 4041–4054. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef]
- Wang, W.T.; Fan, X.Q.; Li, F.H.; Qiu, J.J.; Umair, M.M.; Ren, W.C.; Ju, B.Z.; Zhang, S.F.; Tang, B.T. Magnetochromic Photonic Hydrogel for an Alternating Magnetic Field-Responsive Color Display. Adv. Opt. Mater. 2018, 6, 9. [Google Scholar] [CrossRef]
- Zhang, N.; Lock, J.; Sallee, A.; Liu, H. Magnetic Nanocomposite Hydrogel for Potential Cartilage Tissue Engineering: Synthesis, Characterization, and Cytocompatibility with Bone Marrow Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 20987–20998. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, L.; Wang, Y.; Huang, J.; Wang, Z.; Shi, X.; Zhang, P. An electrically and magnetically responsive nanocomposite of GdPO4·H2O/P3HT/PLGA with electrical stimulation for synergistically enhancing the proliferation and differentiation of pre-osteoblasts. NJCh 2019, 43, 17315–17326. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1568. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Guo, S.; Zheng, Y.; Xu, X.; Meng, H.; Peng, J.; Fang, Z.; Xie, Y. Effects of graphene on the structure, properties, electro-response behaviors of GO/PAA composite hydrogels and influence of electro-mechanical coupling on BMSC differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Carayon, I.; Gaubert, A.; Mousli, Y.; Philippe, B. Electro-responsive hydrogels: Macromolecular and supramolecular approaches in the biomedical field. Biomater. Sci. 2020, 8, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, M.; Zarrintaj, P.; Jafari, S.H.; Hosseini, S.M.; Aliakbari, S.; Pourbadie, H.G.; Naderi, N.; Zibaii, M.I.; Gholizadeh, S.S.; Ramsey, J.D.; et al. Fabricating an electroactive injectable hydrogel based on pluronic-chitosan/aniline-pentamer containing angiogenic factor for functional repair of the hippocampus ischemia rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111328. [Google Scholar] [CrossRef]
- Sgambato, A.; Cipolla, L.; Russo, L. Bioresponsive Hydrogels: Chemical Strategies and Perspectives in Tissue Engineering. Gels 2016, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Ulijn, R.V. Enzyme-responsive materials: A new class of smart biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Zhang, R.; Bowyer, A.; Eisenthal, R.; Hubble, J. A smart membrane based on an antigen-responsive hydrogel. Biotechnol. Bioeng. 2007, 97, 976–984. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef]
- Shen, K.; Sun, G.; Chan, L.; He, L.; Li, X.; Yang, S.; Wang, B.; Zhang, H.; Huang, J.; Chang, M.; et al. Anti-Inflammatory Nanotherapeutics by Targeting Matrix Metalloproteinases for Immunotherapy of Spinal Cord Injury. Small 2021, 17, e2102102. [Google Scholar] [CrossRef]
- Katayama, Y.; Sonoda, T.; Maeda, M. A polymer micelle responding to the protein kinase A signal. Macromolecules 2001, 34, 8569–8573. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Yang, C.; Wang, X. Sensing diffraction gratings of antigen-responsive hydrogel for human immunoglobulin-g detection. Macromol. Rapid Commun. 2010, 31, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.W.; McGeachy, M.J.; Bayir, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [Green Version]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M.S. Regenerative therapies for central nervous system diseases: A biomaterials approach. Neuropsychopharmacology 2014, 39, 169–188. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.M.; Louca, I.; Bolan, F.; Sava, O.R.; Allan, S.M.; Lawrence, C.B.; Pinteaux, E. Regenerative Potential of Hydrogels for Intracerebral Hemorrhage: Lessons from Ischemic Stroke and Traumatic Brain Injury Research. Adv. Healthc. Mater. 2021, 10, e2100455. [Google Scholar] [CrossRef]
- Yao, M.; Chen, Y.; Zhang, J.; Gao, F.; Ma, S.; Guan, F. Chitosan-based thermosensitive composite hydrogel enhances the therapeutic efficacy of human umbilical cord MSC in TBI rat model. Mater. Today Chem. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Dong, G.C.; Kuan, C.Y.; Subramaniam, S.; Zhao, J.Y.; Sivasubramaniam, S.; Chang, H.Y.; Lin, F.H. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 691–699. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Lin, H.H.; Hsu, S.H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Qian, F.; Han, Y.; Han, Z.; Zhang, D.; Zhang, L.; Zhao, G.; Li, S.; Jin, G.; Yu, R.; Liu, H. In Situ implantable, post-trauma microenvironment-responsive, ROS Depletion Hydrogels for the treatment of Traumatic brain injury. Biomaterials 2021, 270, 120675. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Hu, Y.; Li, M.H.; Zhang, Y.C.; Xue, C.C.; Chen, M.H.; Luo, Z.; Cai, K.Y. Remotely-activatable extracellular matrix-mimetic hydrogel promotes physiological bone mineralization for enhanced cranial defect healing. Chem. Eng. J. 2022, 431, 14. [Google Scholar] [CrossRef]
- Adak, A.; Das, G.; Khan, J.; Mukherjee, N.; Gupta, V.; Mallesh, R.; Ghosh, S. Extracellular Matrix (ECM)-Mimicking Neuroprotective Injectable Sulfo-Functionalized Peptide Hydrogel for Repairing Brain Injury. ACS Biomater. Sci. Eng. 2020, 6, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.B.; Danzer, S.C. Impact of Traumatic Brain Injury on Neurogenesis. Front. Neurosci. 2018, 12, 1014. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X.; et al. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021, 131, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Gao, F.; Xu, R.; Zhang, J.; Chen, Y.; Guan, F. A dual-enzymatically cross-linked injectable gelatin hydrogel loaded with BMSC improves neurological function recovery of traumatic brain injury in rats. Biomater. Sci. 2019, 7, 4088–4098. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, G.; Chen, L.; Zhang, Y.; Luo, Y.; Zheng, Y.; Hu, F.; Forouzanfar, T.; Lin, H.; Liu, B. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1alpha promote stem cell differentiation and repair focal brain injury. Bioact. Mater. 2021, 6, 627–637. [Google Scholar] [CrossRef]
- Shi, W.; Huang, C.J.; Xu, X.D.; Jin, G.H.; Huang, R.Q.; Huang, J.F.; Chen, Y.N.; Ju, S.Q.; Wang, Y.; Shi, Y.W.; et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016, 45, 247–261. [Google Scholar] [CrossRef]
- Alvarado-Velez, M.; Enam, S.F.; Mehta, N.; Lyon, J.G.; LaPlaca, M.C.; Bellamkonda, R.V. Immuno-suppressive hydrogels enhance allogeneic MSC survival after transplantation in the injured brain. Biomaterials 2021, 266, 120419. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.Z.; Lee, K.Y.; Qi, Z.X.; Wang, Z.; Xu, Z.Y.; Wu, X.H.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef]
- Maclean, F.L.; Wang, Y.; Walker, R.; Horne, M.K.; Williams, R.J.; Nisbet, D.R. Reducing Astrocytic Scarring after Traumatic Brain Injury with a Multifaceted Anti-Inflammatory Hydrogel System. ACS Biomater. Sci. Eng. 2017, 3, 2542–2549. [Google Scholar] [CrossRef]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 035002. [Google Scholar] [CrossRef] [PubMed]
- Jahanbazi Jahan-Abad, A.; Sahab Negah, S.; Hosseini Ravandi, H.; Ghasemi, S.; Borhani-Haghighi, M.; Stummer, W.; Gorji, A.; Khaleghi Ghadiri, M. Human Neural Stem/Progenitor Cells Derived From Epileptic Human Brain in a Self-Assembling Peptide Nanoscaffold Improve Traumatic Brain Injury in Rats. Mol. Neurobiol. 2018, 55, 9122–9138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Ren, Y.; Guo, S.; Li, J.; Ma, S.; Yao, M.; Guan, F. Injectable hyaluronic acid hydrogel loaded with BMSC and NGF for traumatic brain injury treatment. Mater. Today Bio. 2022, 13, 100201. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Shi, Y.; Pu, H.; Leak, R.K.; Liou, A.K.F.; Badylak, S.F.; Liu, Z.; Zhang, J.; Chen, J.; et al. Implantation of Brain-Derived Extracellular Matrix Enhances Neurological Recovery after Traumatic Brain Injury. Cell Transplant. 2017, 26, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; He, L.; Wu, W. Self-assembling peptide nanofibrous hydrogel as a promising strategy in nerve repair after traumatic injury in the nervous system. Neural. Regen. Res. 2016, 11, 717–718. [Google Scholar] [PubMed]
- Li, J.; Zhang, D.; Guo, S.; Zhao, C.; Wang, L.; Ma, S.; Guan, F.; Yao, M. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol. 2021, 187, 200–213. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, Y.H.; Huang, A.P.; Hsu, S.H. Semi-Interpenetrating Polymer Network of Hyaluronan and Chitosan Self-Healing Hydrogels for Central Nervous System Repair. ACS Appl. Mater. Interfaces 2020, 12, 40108–40120. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Cerpa, W. Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants 2021, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, Y.; He, Y.; Chang, R.; Guo, S.; Ma, S.; Guan, F.; Yao, M. In situ forming and biocompatible hyaluronic acid hydrogel with reactive oxygen species-scavenging activity to improve traumatic brain injury repair by suppressing oxidative stress and neuroinflammation. Mater. Today Bio. 2022, 15, 100278. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-Y.; Lin, Y.-Y.; Chen, C.-Y.; Yang, C.-C.; Chi, C.-Y.; Li, C.-H.; Dong, G.-C.; Lin, F.-H. The preparation of oxidized methylcellulose crosslinked by adipic acid dihydrazide loaded with vitamin C for traumatic brain injury. J. Mater. Chem. B 2019, 7, 4499–4508. [Google Scholar] [CrossRef]
- Louissaint, A.; Rao, S.; Leventhal, C.; Goldman, S.A. Coordinated interaction of Neurogenesis and angiogenesis in the adult songbird brain. Neuron 2002, 34, 945–960. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Guan, F.; Cui, F.; Sun, X.; Zhao, L.; Wang, Y.; Wang, X. Enhanced angiogenesis by the hyaluronic acid hydrogels immobilized with a VEGF mimetic peptide in a traumatic brain injury model in rats. Regen. Biomater. 2019, 6, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Agas, A.; Siddiqui, Z.; Kim, K.; Iglesias-Montoro, P.; Kalluru, J.; Kumar, V.; Haorah, J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact. Mater. 2020, 5, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Chang, K.C.; Chen, L.H.; Liao, S.Y.; Yeh, C.W.; Chuang, Y.J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, X.; Sun, X.; Shen, X.; Yin, H.; Wang, C.; Liu, Y.; Lu, C.; Fu, H.; Yang, S.; et al. Synergistic effects of dual-presenting VEGF- and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale 2019, 11, 19943–19958. [Google Scholar] [CrossRef]





| Molecular Mechanisms | Hydrogels | Components | Loaded Materials | Models | Refs |
|---|---|---|---|---|---|
| Pro-neurogenesis | SA/Col hydrogel | SA, Col | BMSCs, SDF-1 | SD Rats | [76] |
| Gelatin hydrogel | Gelatin, GOX, HRP | BMSCs | SD Rats | [77] | |
| GelMA-IMID hydrogel | Gelatin, MA, IMID | hAMSCs, PDA@SDF-1α | SD Rats | [78] | |
| RADA16-BDNF peptide scaffolds | RADA16, RGI | CXCR4, hUC-MSCs, activated astrocytes | SD Rats | [79] | |
| Agarose hydrogel | Agarose | FasL | SD Rats | [80] | |
| Anti-inflammatory | SA/Col hydrogel | SA, Col | BMSCs, SDF-1 | SD Rats | [76] |
| Fmoc-DIKVAV hydrogel | Fmoc | fucoidan | C57 BL/6 mice | [83] | |
| PEG-bis-AA/HA-DXM hydrogel | PEG, AA, HA | DXM | SD Rats | [84] | |
| BD™PuraMatrix™ hydrogel | PuraMatrix | hNS/PCs, hADSCs | SD Rats | [85] | |
| HA gelatin | HA, GOX, HRP | NGF, BMSCs | C57BL/6 | [86] | |
| ECM hydrogel | ECM | / | C57 BL/6 mice | [87] | |
| Anti-apoptosis | CS-HEC-HA/GP hydrogel | CS, HEC, HA, GP | hUC-MSCs | SD Rats | [69] |
| GH hydrogel | gelatin, HRP, ChOx | BMSCs | C57BL/6 mice | [90] | |
| CH hydrogel | HA, CS | / | SD Rats, Zebrafish | [91] | |
| Anti-oxidation | HT/HGA hydrogel | HA, Tyr | GA | C57BL/6 mice | [93] |
| oxi-MC-ADH-VC hydrogel | MC | Vitamin C | SD Rats | [94] | |
| TM/PC hydrogel | TM, PPS120 | Cur | ICR mice | [72] | |
| C/G/GP hydrogel | CS, gelatin, GP | FA | N2a cells | [70] | |
| Pro-angiogenesis | HA-KLT hydrogel | HA | VEGF mimetic peptide KLT | SD Rats | [96] |
| SAPH | Slanc | / | SD Rats | [97] | |
| SAPH | RADA16-SVVYGLR | / | Zebrafish | [98] | |
| SAPH | RAD | KLT, RGI | SD Rats | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Duan, L.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels 2022, 8, 482. https://doi.org/10.3390/gels8080482
Li X, Duan L, Kong M, Wen X, Guan F, Ma S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels. 2022; 8(8):482. https://doi.org/10.3390/gels8080482
Chicago/Turabian StyleLi, Xingfan, Linyan Duan, Mingyue Kong, Xuejun Wen, Fangxia Guan, and Shanshan Ma. 2022. "Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury" Gels 8, no. 8: 482. https://doi.org/10.3390/gels8080482