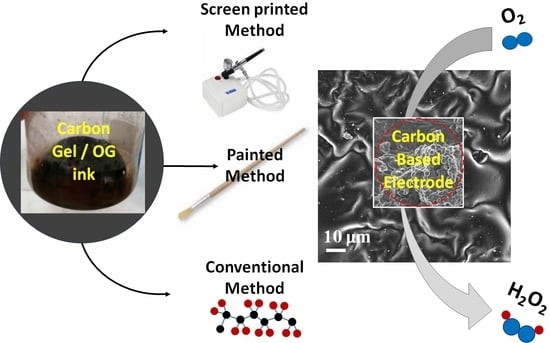
Facile Synthesis of Carbon-Based Inks to Develop Metal-Free ORR Electrocatalysts for Electro-Fenton Removal of Amoxicillin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts ORR
2.1.1. Porosity and Surface Area Determination
2.1.2. Raman Characterization
2.1.3. XPS Characterization
2.1.4. Morphology
2.1.5. Electrochemical Characterization
2.2. Catalyst Fenton
2.2.1. X-ray Diffraction
2.2.2. Morphology
2.3. Electro-Fenton Experiments (EF)
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Materials
4.1.1. Graphene Oxide (OG)
4.1.2. Magnetite (Fe3O4)
4.1.3. Preparation of the Xerogel/OG Ink
4.2. Deposition Methods
4.2.1. Conventional Catalyst (Sample C)
4.2.2. Painted Catalyst (Sample P)
4.2.3. Screen-Printed Catalyst (Sample S)
4.3. Characterization
4.3.1. Chemical and Textural Characterization
4.3.2. Electrochemical Characterization
4.4. Electro-Fenton Processes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, M.; Zhuo, F.; Li, Y.; Yi, P.; Gao, Y.; Zhou, W.; Sun, Y.; Chen, J.; Wu, X.L. Fe-N-C Catalyst Coated on Carbon Felt for Efficient Degradation of Antibiotics via Electro-Fenton Process. Appl. Surf. Sci. 2023, 609, 155310. [Google Scholar] [CrossRef]
- Amarzadeh, M.; Salehizadeh, S.; Damavandi, S.; Mubarak, N.M.; Ghahrchi, M.; Ramavandi, B.; Shahamat, Y.D.; Nasseh, N. Statistical Modeling Optimization for Antibiotics Decomposition by Ultrasound/Electro-Fenton Integrated Process: Non-Carcinogenic Risk Assessment of Drinking Water. J. Environ. Manag. 2022, 324, 116333. [Google Scholar] [CrossRef]
- Li, S.; Hua, T.; Yuan, C.S.; Li, B.; Zhu, X.; Li, F. Degradation Pathways, Microbial Community and Electricity Properties Analysis of Antibiotic Sulfamethoxazole by Bio-Electro-Fenton System. Bioresour. Technol. 2020, 298, 122501. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huang, P.; Chen, X.; Li, L.-P.; Lin, C.-Y.; Chen, X.; Ding, R.; Liu, J.; Chen, R. Ciprofloxacin Degradation Performances and Mechanisms by the Heterogeneous Electro-Fenton with Flocculated Fermentation Biochar. Environ. Pollut. 2023, 324, 121425. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics Degradation by Advanced Oxidation Process (AOPs): Recent Advances in Ecotoxicity and Antibiotic-Resistance Genes Induction of Degradation Products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, H.; Ren, L.; Ou, Y.; Jiang, S.; Chai, Y.; Chen, A.; Yan, B. Treatment of Amoxicillin-Containing Wastewater by Trichoderma Strains Selected from Activated Sludge. Sci. Total Environ. 2023, 867, 161565. [Google Scholar] [CrossRef]
- Armoudjian, Y.; Lin, Q.; Lammens, B.; Daele, J.; Van Annaert, P. Sensitive and Rapid Method for the Quantitation of Amoxicillin in Minipig Plasma and Milk by LC-MS/MS: A Contribution from the IMI ConcePTION Project. J. Pharmacol. Toxicol. Methods 2023, 123, 107264. [Google Scholar] [CrossRef]
- Andrade, L.A.; Souza, G.B.M.; Dias, I.M.; Mour, L.C.; Viana, J.C.V.; Oliveira, S.B.; Alonso, C.G. Degradation of Antibiotic Amoxicillin from Pharmaceutical Industry Wastewater into a Continuous Flow Reactor Using Supercritical Water Gasification. Water Res. 2023, 234, 119826. [Google Scholar] [CrossRef]
- Laksaci, H.; Belhamdi, B.; Khelifi, O.; Khelifi, A. Elimination of Amoxicillin by Adsorption on Coffee Waste Based Activated Carbon. J. Mol. Struct. 2023, 1274, 134500. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Aremu, O.H.; Akintayo, C.O.; Sodeinde, K.O.; Igboama, W.N.; Oseghe, E.O.; Nelana, S.M. Sonocatalytic Degradation of Amoxicillin from Aquaculture Effluent by Zinc Oxide Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100513. [Google Scholar] [CrossRef]
- Li, W.; Zhou, R.; Zhou, R.; Weerasinghe, J.; Zhang, T.; Gissibl, A.; Cullen, P.J.; Speight, R.; Ken, K. Insights into Amoxicillin Degradation in Water by Non-Thermal Plasmas. Chemosphere 2022, 291, 132757. [Google Scholar] [CrossRef] [PubMed]
- Duong-nguyen, T.; Hoang, M.; Hue, N.; Quoc, C. Amoxicillin Degradation Ability of Bacillus Cereus C1 Isolated from Cat Fi Sh Pond Sludge in Vietnam. Heliyon 2022, 8, e11688. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.A.; Han, R.; Qu, L. Occurrence, Detection and Removal of Amoxicillin in Wastewater: A Review. J. Clean. Prod. 2022, 368, 133140. [Google Scholar] [CrossRef]
- Alomar, T.S.; Hameed, B.H.; Usman, M.; Almomani, F.A.; Ba-abbad, M.M.; Khraisheh, M. Recent Advances on the Treatment of Oil Fields Produced Water by Adsorption and Advanced Oxidation Processes. J. Water Process Eng. 2022, 49, 103034. [Google Scholar] [CrossRef]
- Hadi, M.; Rao, R.; Reddy, J. Recent Trends in the Applications of Sonochemical Reactors as an Advanced Oxidation Process for the Remediation of Microbial Hazards Associated with Water and Wastewater: A Critical Review. Ultrason. Sonochem. 2023, 94, 106302. [Google Scholar] [CrossRef]
- Zhai, C.; Chen, Y.; Huang, X.; Isaev, A.B.; Zhu, M. Recent Progress on Single-Atom Catalysts in Advanced Oxidation Processes for Water Treatment. Environ. Funct. Mater. 2022, 1, 219–229. [Google Scholar] [CrossRef]
- Wei, J.; Shi, L.; Wu, X. Electrochemical Advanced Oxidation Process with Simultaneous Persulfate and Hydrogen Peroxide On-Site Generations for High Salinity Wastewater. Sep. Purif. Technol. 2023, 310, 123147. [Google Scholar] [CrossRef]
- Campos, S.; Lorca, J.; Vidal, J.; Calzadilla, W.; Toledo-neira, C.; Aranda, M.; Miralles-cuevas, S.; Cabrera-reina, A.; Salazar, R. Removal of Contaminants of Emerging Concern by Solar Photo Electro-Fenton Process in a Solar Electrochemical Raceway Pond Reactor. Process Saf. Environ. Prot. 2023, 169, 660–670. [Google Scholar] [CrossRef]
- Lai, S.; Zhao, H.; Qu, Z.; Tang, Z.; Yang, X.; Jiang, P. Promotion of Formaldehyde Degradation by Electro-Fenton: Controlling the Distribution of ⋅ OH and Formaldehyde near Cathode to Increase the Reaction Probability. Chemosphere 2022, 307, 135776. [Google Scholar] [CrossRef]
- Wang, J.; Qin, J.; Liu, B.; Song, S. Reaction Mechanisms and Toxicity Evolution of Sulfamethoxazole Degradation by CoFe-N Doped C as Electro-Fenton Cathode. Sep. Purif. Technol. 2022, 298, 121655. [Google Scholar] [CrossRef]
- Tu, S.; Ning, Z.; Duan, X.; Zhao, X.; Chang, L. Efficient Electrochemical Hydrogen Peroxide Generation Using TiO2/RGO Catalyst and Its Application in Electro-Fenton Degradation of Methyl Orange. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 651, 129657. [Google Scholar] [CrossRef]
- Fajardo-Puerto, E.; Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. From Fenton and ORR 2e−Type Catalysts to Bifunctional Electrodes for Environmental Remediation Using the Electro-Fenton Process. Catalysts 2023, 13, 674. [Google Scholar] [CrossRef]
- Qin, X.; Wang, K.; Cao, P.; Su, Y.; Chen, S.; Yu, H.; Quan, X. Highly Efficient Electro-Fenton Process on Hollow Porous Carbon Spheres Enabled by Enhanced H2O2 Production and Fe2+ Regeneration. J. Hazard. Mater. 2023, 446, 130664. [Google Scholar] [CrossRef] [PubMed]
- Garza-Campos, B.; Morales-Acosta, D.; Hernández-Ramírez, A.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Manríquez, J.; Ruiz-Ruiz, E.J. Air Diffusion Electrodes Based on Synthetized Mesoporous Carbon for Application in Amoxicillin Degradation by Electro-Fenton and Solar Photo Electro-Fenton. Electrochim. Acta 2018, 269, 232–240. [Google Scholar] [CrossRef]
- Molla, H.; Pishnamaz, N.; Farimaniraad, H.; Baghdadi, M.; Aminzadeh, B.; Mahpishanian, S. Application of Nickel Foam Cathode Modi Fi Ed by Single-Wall Carbon Nanotube in Electro-Fenton Process Coupled with Anodic Oxidation: Enhancing Organic Pollutants Removal. J. Electroanal. Chem. 2023, 929, 117130. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Gao, J.; Meng, H.; Chai, S.; Jian, Y.; Shi, L.; Wang, Y.; He, C. Selective Electrochemical H2O2 Generation on the Graphene Aerogel for Efficient Electro-Fenton Degradation of Ciprofloxacin. Sep. Purif. Technol. 2021, 272, 118884. [Google Scholar] [CrossRef]
- Wang, Z.; Olvera-vargas, H.; Vinicius, M.; Martins, S.; Garcia-rodriguez, O.; Garaj, S.; Lefebvre, O. High Performance and Durable Graphene-Grafted Cathode for Electro-Fenton Degradation of Tetramethyldecynediol. Chem. Eng. J. 2023, 455, 140643. [Google Scholar] [CrossRef]
- Ramírez-Valencia, L.D.; Bailón-García, E.; Moral-Rodríguez, A.I.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Carbon Gels–Green Graphene Composites as Metal-Free Bifunctional Electro-Fenton Catalysts. Gels 2023, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Briz-Amate, T.; Castelo-Quibén, J.; Bailón-García, E.; Abdelwahab, A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Growing Tungsten Nanophases on Carbon Spheres Doped with Nitrogen. Behaviour as Electro-Catalysts for Oxygen Reduction Reaction. Materials 2021, 14, 7716. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Ros, C.H.; Morales-Torres, S.; Pérez-Cadenas, M.; Kooyman, P.J.; Moreno-Castilla, C.; Kapteijn, F. Metal-Doped Carbon Xerogels for the Electro-Catalytic Conversion of CO2 to Hydrocarbons. Carbon N. Y. 2013, 56, 324–331. [Google Scholar] [CrossRef]
- Wang, S.; Ye, D.; Zhu, X.; Yang, Y.; Chen, J.; Liu, Z.; Chen, R.; Liao, Q. Beyond the Catalyst: A Robust and Omnidirectional Hydrophobic Triple-Phase Architecture for Ameliorating Air-Breathing H2O2 Electrosynthesis and Wastewater Remediation. Sep. Purif. Technol. 2023, 305, 122397. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, Y.; Wu, J.; Feng, Z.; Feng, F.; Li, Y.; Yang, Q. Development of a Three-Dimensional Electro-Fenton System Packed with C-PTFE/Fe–Co–C Hybrid Particle Electrodes for Simultaneous H2O2 Generation and Activation into • OH. Sep. Purif. Technol. 2023, 317, 123960. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated Carbons from KOH and H3PO4-Activation of Olive Residues and Its Application as Supercapacitor Electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Aoki, A.; Ogasawara, T.; Aoki, T.; Ishida, Y.; Shimamura, Y. Raman Spectroscopy Used for Estimating the Effective Elastic Modulus of Carbon Nanotubes in Aligned Multi-Walled Carbon Nanotubes / Epoxy Composites under Tensile Loading. Compos. Part A 2023, 167, 107448. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.; Couzi, M. Vibrational Spectroscopy Raman Spectroscopy as a Tool for the Analysis of Carbon-Based Materials (Highly Oriented Pyrolitic Graphite, Multilayer Graphene and Multiwall Carbon Nanotubes) and of Some of Their Elastomeric Composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman Spectroscopy of Carbon Materials and Their Composites: Graphene, Nanotubes and Fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, W.; Du, X.; Yang, X.; Wang, F.; Zhou, Y.; Pan, Y.; Lu, Y. Materials Today Sustainability An N, P, O-Doped Porous Carbon Electrode Material Derived from a Lignin-Modi Fi Ed Chitosan Xerogel for a Supercapacitor. Mater. Today Sustain. 2023, 22, 100372. [Google Scholar] [CrossRef]
- Medina, O.E.; Galeano-caro, D.; Ocampo-p, R.; Carrasco-marín, F.; Franco, C.A.; Corte, F.B. Microporous and Mesoporous Materials Development of a Monolithic Carbon Xerogel-Metal Composite for Crude Oil Removal from Oil in-Saltwater Emulsions: Evaluation of Reuse Cycles. Microporous Mesoporous Mater. 2021, 327, 111424. [Google Scholar] [CrossRef]
- Gaikwad, M.M.; Sarode, K.K.; Pathak, A.D.; Sharma, C.S. Ultrahigh Rate and High-Performance Lithium-Sulfur Batteries with Resorcinol-Formaldehyde Xerogel Derived Highly Porous Carbon Matrix as Sulfur Cathode Host. Chem. Eng. J. 2021, 425, 131521. [Google Scholar] [CrossRef]
- Perciani, N.; Moraes, D.; Siervo, A.D.; Moreira, T.; Campos, B.; Thim, P.; Alvares, L. Structure-Directing Ability of the Kraft-Lignin / Cellulose Carbon Xerogel for the Development of C-Nb2O5 Sunlight-Active Photocatalysts. J. Photochem. Photobiol. A Chem. 2023, 441, 114697. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Wang, W.; Fortner, J. Organic Functionalized Graphene Oxide Behavior in Water. Nanomaterials 2020, 10, 1228. [Google Scholar] [CrossRef]
- Beeran, Y.; Bobnar, V.; Grohens, Y.; Thomas, S.; Kokol, V. Cellulose Nano Fi Brils-Reduced Graphene Oxide Xerogels and Cryogels for Dielectric and Electrochemical Storage Applications z Fin. Polymer 2018, 147, 260–270. [Google Scholar] [CrossRef]
- Pham, H.D.; Pham, V.H.; Cuong, T.V. Synthesis of the Chemically Converted Graphene Xerogel with Superior Electrical Conductivity, W. ChemComm 2011, 47, 9672–9674. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Li, Y.; Zhang, Q.; Chen, H.; Zhang, J.; Huang, T. Anthraquinone (AQS)/Polyaniline (PANI) Modified Carbon Felt (CF) Cathode for Selective H2O2 Generation and Efficient Pollutant Removal In. J. Environ. Manag. 2022, 304, 114315. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Liu, H.; Hu, X.; Zhou, S. Insight into the Mechanisms of BPS Degradation by Electro-Fenton Method Modified by Co-Based Nanoparticles on the Oxidized Carbon Cathode. Chem. Eng. J. 2022, 446, 137376. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Yang, F.; Che, S.; Chen, N.; Xu, C.; Wu, N.; Sun, Y.; Yu, C.; Li, Y. Multifunctional Electrocatalyst Based on MoCoFe LDH Nanoarrays for the Coupling of High Efficiency Electro-Fenton and Water Splitting Process. Chem. Eng. J. 2023, 467, 143274. [Google Scholar] [CrossRef]
- Wang, L.; Niu, J.; Gao, S.; Liu, Z.; Wu, S.; Huang, M.; Li, H.; Zhu, M.; Yuan, R. Breakthrough in Controlling Membrane Fouling and Complete Demulsification via Electro-Fenton Pathway: Principle and Mechanisms. J. Memb. Sci. 2023, 670, 121354. [Google Scholar] [CrossRef]
- Zhao, H.; Han, S.; Jia, J.; He, M.; An, K.; Tang, Z.; Lai, S.; Yang, X.; Wang, Z. The Effect of N-Doping on the Synergy between Adsorption and 2e-ORR Performance of Activated Carbon Cathode in an Electro-Fenton System. Chem. Eng. J. 2023, 468, 143505. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, R.; Chi, C.; Chen, X.; Pan, Y.; Li, J.; Xia, X. One-Step Synthesis of S, N Dual-Element Doped RGO as an Ef Fi Cient Electrocatalyst for ORR. J. Electroanal. Chem. 2023, 940, 117489. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Smirnova, D.N.; Noskov, A.V.; Yu, O.; Kirilenko, M.A.; Agafonov, A.V. Mesoporous Halloysite/Magnetite Composite: Synthesis, Characterization and in Vitro Evaluation of the Effect on the Bacteria Viability. Mater. Today Commun. 2022, 32, 103877. [Google Scholar] [CrossRef]
- Iglesias-rojas, D.; Bar, A.; Muro, I.G.D.; Lezama, L.; Insausti, M. Getting Insight into How Iron (III) Oleate Precursors Affect the Features of Magnetite Nanoparticles. J. Solid State Chem. 2022, 316, 123619. [Google Scholar] [CrossRef]
- Vinod, K.R.; Saravanan, P.; Sakar, M.; Balakumar, S. Insights into the Nitridation of Zero-Valent Iron Nanoparticles for the Facile Synthesis of Iron Nitride Nanoparticles. RSC Adv. 2016, 123619, 45850–45857. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. The Effect of Vacuum Annealing of Magnetite and Zero-Valent Iron Nanoparticles on the Removal of Aqueous Uranium. J. Nanotechnol. 2013, 2013, 173625. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, R.; Yang, J.; Wang, P. Enhanced Heterogeneous Fenton Degradation of Organic Pollutants by CRC/Fe3O4 Catalyst at Neutral PH. Front. Chem. 2022, 10, 892424. [Google Scholar] [CrossRef]
- Hashemian, S.; Tabatabaee, M.; Gafari, M. Fenton Oxidation of Methyl Violet in Aqueous Solution. J. Chem. 2013, 2013, 509097. [Google Scholar] [CrossRef]
- Li, A.; Zhang, M.; Ma, W.; Li, D.; Xu, Y. Sugar-Disguised Bullets for Combating Multidrug-Resistant Bacteria Infections Based on an Oxygen Vacancy-Engineered Glucose-Functionalized MoO3-x Photo-Coordinated Bienzyme. Chem. Eng. J. 2022, 431, 133943. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Song, C.; Tang, D.; Sun, J. Double-Potential Electro-Fenton: A Novel Strategy Coupling Oxygen Reduction Reaction and Fe2+/Fe3+ Recycling. Electrochem. Commun. 2018, 94, 55–58. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.; Senthilnathan, J. Ferrocene Functionalized Graphene Based Electrode for the Electro−Fenton Oxidation of Ciprofloxacin. Chemosphere 2018, 209, 113–123. [Google Scholar] [CrossRef]
- Company, L.; William, B.; Offeman, R.E. Preparation of Graphitic Oxide. J. Chem. 1958, 89, 1958. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and Application of Magnetic Fe3O4 Nanoparticles for Wastewater Purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Bailón-garcía, E.; Carrasco-marín, F.; Pérez-cadenas, A.F.; Maldonado-hódar, F.J. Chemoselective Pt-Catalysts Supported on Carbon-TiO2 Composites for the Direct Hydrogenation of Citral to Unsaturated Alcohols. J. Catal. 2016, 344, 701–711. [Google Scholar] [CrossRef]
- Fernández-Sáez, N.; Villela-Martinez, D.E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Pastrana-Martínez, L.M. Heteroatom-Doped Graphene Aerogels and Carbon-Magnetite Catalysts for the Heterogeneous Electro-Fenton Degradation of Acetaminophen in Aqueous Solution. J. Catal. 2019, 378, 68–79. [Google Scholar] [CrossRef]






 S,
S,  C and
C and  P.
P.
 S,
S,  C and
C and  P.
P.






| Sample | SB.E.T | SDFT | SDR | W0 | L0 | WDFT | LDFT | V0.95 | Vmeso |
|---|---|---|---|---|---|---|---|---|---|
| m2 g−1 | m2 g−1 | m2 g−1 | cm3 g−1 | nm | cm3 g−1 | nm | cm3 g−1 | cm3 g−1 | |
| S | 436 | 506 | 492.2 | 0.170 | 0.63 | 0.19 | 0.61 | 0.218 | 0.043 |
| C | 383 | 448 | 424.2 | 0.150 | 0.60 | 0.17 | 0.61 | 0.171 | 0.020 |
| P | 363 | 428 | 408.6 | 0.145 | 0.65 | 0.15 | 0.61 | 0.183 | 0.038 |
| Bond | Peak | S (%) | C (%) | P (%) |
|---|---|---|---|---|
| C-C | 284.6 | 70.3 | 68.8 | 71.2 |
| C-O | 285.4 | 18.7 | 19.7 | 18.9 |
| C-O-C | 286.8 | 5.5 | 5.7 | 5.3 |
| C=O | 288.5 | 3.3 | 3.6 | 2.7 |
| CO2 | 290.7 | 2.1 | 2.2 | 1.9 |
| Total C | --- | 93.8 | 93.0 | 94.1 |
| Total O2 | --- | 6.2 | 7.0 | 5.9 |
| Sample | JK mA cm−2 | n | E° Initial V |
|---|---|---|---|
| S | 14.35 | 2.33 | −0.25 |
| C | 9.00 | 2.50 | −0.23 |
| P | 14.59 | 2.03 | −0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Valero, L.C.; Fajardo-Puerto, E.; Elmouwahidi, A.; Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Facile Synthesis of Carbon-Based Inks to Develop Metal-Free ORR Electrocatalysts for Electro-Fenton Removal of Amoxicillin. Gels 2024, 10, 53. https://doi.org/10.3390/gels10010053
Valencia-Valero LC, Fajardo-Puerto E, Elmouwahidi A, Bailón-García E, Carrasco-Marín F, Pérez-Cadenas AF. Facile Synthesis of Carbon-Based Inks to Develop Metal-Free ORR Electrocatalysts for Electro-Fenton Removal of Amoxicillin. Gels. 2024; 10(1):53. https://doi.org/10.3390/gels10010053
Chicago/Turabian StyleValencia-Valero, Laura Carolina, Edgar Fajardo-Puerto, Abdelhakim Elmouwahidi, Esther Bailón-García, Francisco Carrasco-Marín, and Agustín Francisco Pérez-Cadenas. 2024. "Facile Synthesis of Carbon-Based Inks to Develop Metal-Free ORR Electrocatalysts for Electro-Fenton Removal of Amoxicillin" Gels 10, no. 1: 53. https://doi.org/10.3390/gels10010053