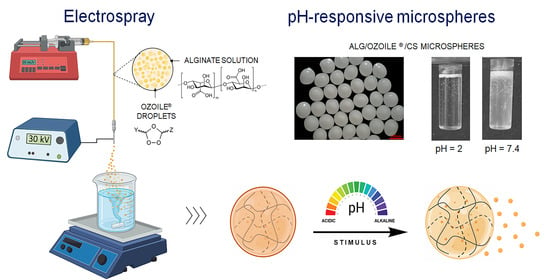
Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of MS Fabrication by Electrospray
2.2. Morphology and Swelling Properties of Alginate/Ozoile MS
2.3. FTIR Analysis of Electrosprayed Alginate/Ozoile MS
2.4. Thermal Analysis
2.5. pH-Dependent Degradation of Alginate/Ozoile MS
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Gel Microspheres
4.3. Morphological and Swelling Analyses
4.4. FTIR Analysis
4.5. Thermal Characterization
4.6. Degradation Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciarleglio, G.; Cinti, F.; Toto, E.; Santonicola, M.G. Synthesis and Characterization of Alginate Gel Beads with Embedded Zeolite Structures as Carriers of Hydrophobic Curcumin. Gels 2023, 9, 714. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Vella, S.; Toto, E.; Santonicola, M.G. Emulsion-based multi-responsive microspheres for the delivery of lipophilic Ozoile. Ceram. Int. 2023, 49, 24517–24524. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Paciello, A.; Santonicola, M.G. A supramolecular two-photon-active hydrogel platform for direct bioconjugation under near-infrared radiation. J. Mater. Chem. B 2015, 3, 1313–1320. [Google Scholar] [CrossRef]
- Paciello, A.; Santonicola, M.G. Supramolecular polycationic hydrogels with high swelling capacity prepared by partial methacrylation of polyethyleneimine. RSC Adv. 2015, 5, 88866–88875. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2021, 170, 424–436. [Google Scholar] [CrossRef]
- Kang, S.-M.; Lee, J.-H.; Huh, Y.S.; Takayama, S. Alginate Microencapsulation for Three-Dimensional In Vitro Cell Culture. ACS Biomater. Sci. Eng. 2021, 7, 2864–2879. [Google Scholar] [CrossRef]
- Silva, S.; Fernandes, E.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.; Reis, R. 2.11 Polymers of Biological Origin. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 228–252. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liao, I.C.; Adler, A.; Leong, K.W. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv. Drug Deliv. Rev. 2009, 61, 1043–1054. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Huang, Y.-T.; Weng, C.-J.; Chen, C.-M.; Su, Y.-F.; Chu, S.-Y.; Tseng, J.-H.; Wu, R.-C.; Liu, S.-J. Preparation and in vitro/in vivo evaluation of doxorubicin-loaded poly[lactic-co-glycol acid] microspheres using electrospray method for sustained drug delivery and potential intratumoral injection. Colloids Surf. B Biointerfaces 2020, 190, 110937. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, W.; Zheng, J.; Wang, Q.; Ma, G.; Liu, H.; Wang, X. Hierarchically electrospraying a PLGA@chitosan sphere-in-sphere composite microsphere for multi-drug-controlled release. Regen. Biomater. 2020, 7, 381–390. [Google Scholar] [CrossRef]
- Russo, T.; Currò, M.; Ferlazzo, N.; Caccamo, D.; Perrone, P.; Arena, S.; Antonelli, E.; Antonuccio, P.; Ientile, R.; Romeo, C.; et al. Stable Ozonides with Vitamin E Acetate versus Corticosteroid in the Treatment of Lichen Sclerosus in Foreskin: Evaluation of Effects on Inflammation. Urol. Int. 2019, 103, 459–465. [Google Scholar] [CrossRef]
- Kuczkowski, R.L. The structure and mechanism of formation of ozonides. Chem. Soc. Rev. 1992, 21, 79–83. [Google Scholar] [CrossRef]
- Currò, M.; Russo, T.; Ferlazzo, N.; Caccamo, D.; Antonuccio, P.; Arena, S.; Parisi, S.; Perrone, P.; Ientile, R.; Romeo, C.; et al. Anti-Inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans. Molecules 2018, 23, 645. [Google Scholar] [CrossRef]
- Bertuccio, M.P.; Rizzo, V.; Arena, S.; Trainito, A.; Montalto, A.S.; Caccamo, D.; Currò, M.; Romeo, C.; Impellizzeri, P. Ozoile Reduces the LPS-Induced Inflammatory Response in Colonic Epithelial Cells and THP-1 Monocytes. Curr. Issues Mol. Biol. 2023, 45, 1333–1348. [Google Scholar] [CrossRef]
- Zagolin, G.; Loss, G.; Tasinato, M.; Cassino, R.; Degli Angeli, G.; Tasinato, R. Advantages of the Topical Application of Ozoile in the Healing of Venous Ulcers of the Lower Limbs. A Randomized Clinical Study. BJSTR 2022, 47, 38603–38608. [Google Scholar] [CrossRef]
- Ricci, E.; Pittarello, M.; Giacinto, F. Studio di valutazione del presidio Rigenoma con ozoile nel trattamento di lesioni cutanee croniche. Ital. J. Wound Care 2022, 6, 31–35. [Google Scholar] [CrossRef]
- Saralidze, K.; Koole, L.H.; Knetsch, M.L. Polymeric microspheres for medical applications. Materials 2010, 3, 3537–3564. [Google Scholar] [CrossRef]
- Raut, S.Y.; Gahane, A.; Joshi, M.B.; Kalthur, G.; Mutalik, S. Nanocomposite clay-polymer microbeads for oral controlled drug delivery: Development and, in vitro and in vivo evaluations. J. Drug Deliv. Sci. Technol. 2019, 51, 234–243. [Google Scholar] [CrossRef]
- Raja, N.; Park, H.; Choi, Y.-J.; Yun, H.-s. Multifunctional Calcium-Deficient Hydroxyl Apatite–Alginate Core–Shell-Structured Bone Substitutes as Cell and Drug Delivery Vehicles for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Georgiev, V.F.; Batakliev, T.T.; Anachkov, M.P.; Rakovski, S.K. Study of Ozonated Olive Oil: Monitoring of the Ozone Absorption and Analysis of the Obtained Functional Groups. Ozone Sci. Eng. 2015, 37, 55–61. [Google Scholar] [CrossRef]
- Beşen, B.S.; Balcı, O.; Güneşoğlu, C.; Orhan, M.; İnci Somuncuoğlu, E.; İrem Tatlı, İ. Obtaining medical textiles including microcapsules of the ozonated vegetable oils. Fibers Polym. 2017, 18, 1079–1090. [Google Scholar] [CrossRef]
- John, J.; Bhattacharya, M.; Raynor, P.C. Emulsions containing vegetable oils for cutting fluid application. Colloids Surf. A Physicochem. Eng. 2004, 237, 141–150. [Google Scholar] [CrossRef]
- Beşen, B.S.; Balci, O. Antibacterial Finishing of 100% Cotton Fabric with β-Cyclodextrin-Ozonated Olive Oil Inclusion Complex. AATCC J. Res. 2016, 3, 12–18. [Google Scholar] [CrossRef]
- Tarhan, İ.; Ismail, A.A.; Kara, H. Quantitative determination of free fatty acids in extra virgin olive oils by multivariate methods and Fourier transform infrared spectroscopy considering different absorption modes. Int. J. Food Prop. 2017, 20, 790–797. [Google Scholar] [CrossRef]
- Alshuiael, S.M.; Al-Ghouti, M.A. Multivariate analysis for FTIR in understanding treatment of used cooking oil using activated carbon prepared from olive stone. PLoS ONE 2020, 15, e0232997. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res. Int. 2010, 43, 886–892. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Alexa, E.; Munteanu, M.-F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Sagdicoglu Celep, A.G.; Demirkaya, A.; Solak, E.K. Antioxidant and anticancer activities of gallic acid loaded sodium alginate microspheres on colon cancer. Curr. Appl. Phys. 2022, 40, 30–42. [Google Scholar] [CrossRef]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Drosos, A.; Boura, K.; Dima, A.; Karabagias, I.K.; Nigam, P.S.; Kanellaki, M.; Koutinas, A.A. Consolidated bioprocessing of starch based on a bilayer cell factory without genetic modification of yeast. Environ. Technol. Innov. 2021, 24, 101844. [Google Scholar] [CrossRef]
- Chiavaro, E.; Vittadini, E.; Rodriguez-Estrada, M.T.; Cerretani, L.; Bonoli, M.; Bendini, A.; Lercker, G. Monovarietal extra virgin olive oils: Correlation between thermal properties and chemical composition. J. Agric. Food Chem. 2007, 55, 10779–10786. [Google Scholar] [CrossRef]
- Lorenzo, C.; Alessandra, B.; Massimiliano, R.; Maria, P.; Stefano, V.; Emma, C. DSC evaluation of extra virgin olive oil stability under accelerated oxidative test: Effect of fatty acid composition and phenol contents. J. Oleo Sci. 2012, 61, 303–309. [Google Scholar] [CrossRef]
- Tan, C.; Che Man, Y. Differential scanning calorimetric analysis of edible oils: Comparison of thermal properties and chemical composition. JAOCS 2000, 77, 143–155. [Google Scholar] [CrossRef]
- Lee, B.-B.; Ravindra, P.; Chan, E.-S. Size and Shape of Calcium Alginate Beads Produced by Extrusion Dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Smrdel, P.; Bogataj, M.; Mrhar, A. The Influence of Selected Parameters on the Size and Shape of Alginate Beads Prepared by Ionotropic Gelation. Sci. Pharm. 2008, 76, 77–90. [Google Scholar] [CrossRef]
- Chan, E.-S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and Thermo-Responsive Composite Hydrogels with Poly(N-isopropylacrylamide) and Carbon Nanotubes Fabricated by Two-Step Photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef]
- Dunmur, R.; Murray, M. Spectroscopic Methods in Organic Chemistry, 2nd ed.; Hesse, M., Meier, H., Zeeh, B., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2008. [Google Scholar] [CrossRef]
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch Equation: Its History and Limitations. J. Chem. Educ. 2001, 78, 1499. [Google Scholar] [CrossRef]








| Flow Rate (mL/h) | Sample | Needle Size 0.311 mm (24 G) | Needle Size 0.514 mm (21 G) | ||
|---|---|---|---|---|---|
| 25 kV | 30 kV | 25 kV | 30 kV | ||
| Mean Diameter (μm) | Mean Diameter (μm) | Mean Diameter (μm) | Mean Diameter (μm) | ||
| 20 | No coating | 311 ± 25 | 279 ± 21 | 456 ± 21 | 375 ± 37 |
| Coating | 265 ± 24 | 249 ± 27 | 359 ± 45 | 331 ± 37 | |
| 30 | No coating | 353 ± 32 | 328 ± 39 | 705 ± 50 | 428 ± 18 |
| Coating | 306 ± 19 | 268 ± 28 | 663 ± 61 | 372 ± 24 | |
| Source of Variation | % of Total Variation | SS | DF | MS | F Ratio | p Value | Significant? |
|---|---|---|---|---|---|---|---|
| Needle size | 44.38 | 3,001,037 | 1 | 3,001,037 | F = 2965 | <0.0001 | Yes |
| Flow rate | 14.22 | 961,969 | 1 | 961,969 | F = 950.3 | <0.0001 | Yes |
| Applied voltage | 15.96 | 1,079,521 | 1 | 1,079,521 | F = 1066 | <0.0001 | Yes |
| Needle size × Flow rate | 4.082 | 276,045 | 1 | 276,045 | F = 272.7 | <0.0001 | Yes |
| Needle size × Applied voltage | 8.436 | 570,478 | 1 | 570,478 | F = 563.6 | <0.0001 | Yes |
| Flow rate × Applied voltage | 3.235 | 218,790 | 1 | 218,790 | F = 216.1 | <0.0001 | Yes |
| Needle size × Flow rate × Applied voltage | 3.817 | 258,115 | 1 | 258,115 | F = 255.0 | <0.0001 | Yes |
| Flow Rate (mL/h) | Sample | Needle Size 0.311 mm (24 G) | Needle Size 0.514 mm (21 G) | ||
|---|---|---|---|---|---|
| 25 kV | 30 kV | 25 kV | 30 kV | ||
| SF | SF | SF | SF | ||
| 20 | No coating | 0.041 ± 0.035 | 0.025 ± 0.021 | 0.019 ± 0.015 | 0.040 ± 0.029 |
| Coating | 0.044 ± 0.033 | 0.038 ± 0.026 | 0.056 ± 0.044 | 0.039 ± 0.034 | |
| 30 | No coating | 0.023 ± 0.018 | 0.019 ± 0.014 | 0.012 ± 0.010 | 0.029 ± 0.021 |
| Coating | 0.036 ± 0.051 | 0.040 ± 0.038 | 0.048 ± 0.035 | 0.040 ± 0.031 | |
| Source of Variation | % of Total Variation | SS | DF | MS | F Ratio | p Value | Significant? |
|---|---|---|---|---|---|---|---|
| Needle size | 0.1797 | 0.0004000 | 1 | 0.0004000 | F = 0.8437 | 0.3589 | No |
| Flow rate | 4.954 | 0.01103 | 1 | 0.01103 | F = 23.25 | <0.0001 | Yes |
| Applied voltage | 0.9099 | 0.002025 | 1 | 0.002025 | F = 4.271 | 0.0394 | Yes |
| Needle size × Flow rate | 0.1011 | 0.0002250 | 1 | 0.0002250 | F = 0.4746 | 0.4913 | No |
| Needle size × Applied voltage | 9.447 | 0.02103 | 1 | 0.02103 | F = 44.34 | <0.0001 | Yes |
| Flow rate × Applied voltage | 0.1797 | 0.0004000 | 1 | 0.0004000 | F = 0.8437 | 0.3589 | No |
| Needle size × Flow rate × Applied voltage | 0.7189 | 0.001600 | 1 | 0.001600 | F = 3.375 | 0.0670 | No |
| rpm | Mean Diameter (μm) | Water Content (%) | Swelling Ratio |
|---|---|---|---|
| 500 | 305 ± 28 | 84.18 ± 0.48 | 6.33 ± 0.19 |
| 550 | 295 ± 24 | 79.75 ± 0.39 | 4.94 ± 0.09 |
| 600 | 290 ± 19 | 81.85 ± 0.51 | 5.51 ± 0.15 |
| 650 | 262 ± 30 | 80.52 ± 0.71 | 5.14 ± 0.19 |
| 700 | 249 ± 27 | 82.19 ± 0.92 | 5.62 ± 0.31 |
| Ozoile (wt%) | Mean Diameter (μm) | |
|---|---|---|
| No Coating | Coating | |
| 0 | 296 ± 27 | 277 ± 18 |
| 10 | 257 ± 15 | 238 ± 21 |
| 20 | 279 ± 21 | 249 ± 27 |
| 30 | 286 ± 12 | 267 ± 22 |
| 40 | 299 ± 18 | 284 ± 30 |
| 50 | 326 ± 32 | 313 ± 28 |
| Frequency (cm−1) | Functional Group Vibration | Peak Intensity | Ref. | |
|---|---|---|---|---|
| EVO Oil | Ozoile | |||
| 3600 | O–H stretching | - | m | [25] |
| 3005 | =C–H stretching | m | w | [27] |
| 2954 | Asymmetric stretching vibration of methyl (–CH3) group | m | w | [26] |
| 2924 and 2852 | Asymmetric and symmetric stretching vibration of methylene (–CH2) group | m | s | [28] |
| 1743 | Carbonyl (C=O) from the ester linkage of triacylglycerol | s | s | [29] |
| 1654 | cis C=C | s | m | [27] |
| 1464 | Bending vibrations of the CH2 and CH3 aliphatic groups | s | s | [27] |
| 1417 | Rocking vibrations of CH bonds of cis-disubstituted alkenes | m | w | [30] |
| 1377 | Symmetric bending vibrations of CH3 groups | w | s | [30] |
| 1240 | vibrations of stretching mode from the C–O group in esters | w | m | [30] |
| 1161 | vibrations of stretching mode from the C–O group in esters | w | s | [30] |
| 1097 | –CH bending and –CH deformation vibrations of fatty acids | w | w | [31] |
| 1033 | C–O stretching | w | m | [27] |
| 966 | bending vibration of CH functional groups of isolated trans-olefin | w | w | [30] |
| 907 | Bending vibration of cis –HC=CH– | w | - | [31] |
| 721 | Overlapping of the methylene (–CH2) rocking vibration and to the out of plane vibration of cis-disubstituted olefins | s | s | [30] |
| Sample | TC | ΔH | TC′ | ΔH |
|---|---|---|---|---|
| EVO Oil | −40.35 ± 0.25 | −28.71 ± 1.06 | −21.22 ± 0.70 | −2.69 ± 0.85 |
| Ozoile | −54.16 ± 0.18 | −6.70 ± 0.36 | −11.72 ± 1.60 | −4.83 ± 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarleglio, G.; Russo, T.; Toto, E.; Santonicola, M.G. Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process. Gels 2024, 10, 52. https://doi.org/10.3390/gels10010052
Ciarleglio G, Russo T, Toto E, Santonicola MG. Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process. Gels. 2024; 10(1):52. https://doi.org/10.3390/gels10010052
Chicago/Turabian StyleCiarleglio, Gianluca, Tiziana Russo, Elisa Toto, and Maria Gabriella Santonicola. 2024. "Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process" Gels 10, no. 1: 52. https://doi.org/10.3390/gels10010052