Diversity, Lifestyle, Genomics, and Their Functional Role of Cochliobolus, Bipolaris, and Curvularia Species in Environmental Remediation and Plant Growth Promotion under Biotic and Abiotic Stressors
Abstract
:1. Introduction
2. Diversity, Habitation, and Ecology of Cochliobolus, Curvularia, and Bipolaris
3. Classification, Nomenclature, and Phylogenetic Analysis
4. Cultivation and Preservation in Culture
5. The Pleomorphic Genus Cochliobolus
6. Morphology of Curvularia and Bipolaris
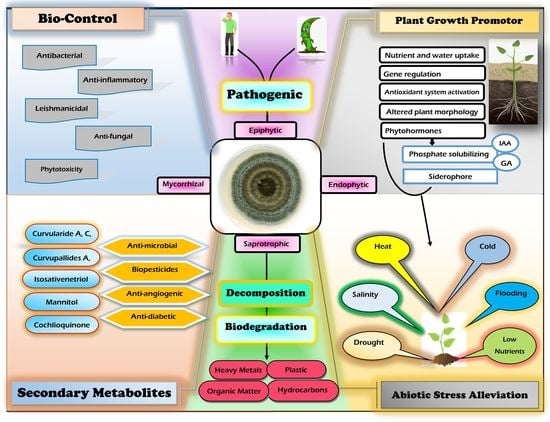
7. Cochliobolus, Curvularia and Bipolaris Lifestyle
7.1. Cochliobolus, Curvularia and Bipolari as Epiphytes
7.2. Cochliobolus, Curvularia, and Bipolari as Endophytes
7.3. Cochliobolus, Curvularia, and Bipolaris as Saprobes
8. Application of Cochliobolus, Curvularia, and Bipolaris in Agriculture and Functional Roles
8.1. As a Plant Growth Promotor
8.2. Phytohormone Production
8.3. Phosphate-Solubilizing Agent
9. Bipolaris and Curvularia Secondary Metabolite Production
10. Biological Activities of the Genera Curvularia and Bipolaris
11. Role of Cochliobolus, Curvularia, and Bipolaris Species in Abiotic Stress Tolerance
11.1. Salinity Stress Tolerance
11.2. Enhance Heat and Drought Stress Tolerance
12. Curvularia and Bipolaris Biotechnological Applications
12.1. As a Biocontrol Agent
12.2. Cochliobolus, Curvularia, and Bipolaris Species in Enzyme Production and Biotransformation
13. In Bioremediation and Waste Biomass Valorization
14. Genomics of Curvularia and Bipolaris Species
15. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IAA | Indole acetic acid |
| GA | Gibberellic acid |
| PCB | Polychlorinated biphenyls |
| PAH | Polyaromatic hydrocarbons |
| rDNA | Ribosomal deoxyribonucleic acid |
| ITS | Internal transcribed spacer |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| NCBI | National Center for Biotechnology Information |
| ML | Maximum likelihood |
| sp | Species |
| P | Phosphorus |
| MIC | Minimum inhibitory concentration |
| RNA | Ribonucleic acid |
| cThTv | Curvularia thermal tolerance virus |
| POME | Palm oil mill effluent |
| LME | Lignin-modifying enzyme |
| ROS | Reactive oxygen species |
| USD | United States Dollar |
| Mg | Milligram |
| L | Liter |
| NGS | Next-generation sequencing |
| GC | Guanine–cytosine |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| Cas9 | CRISPR-associated protein 9 |
References
- Yuvaraj, M.; Ramasamy, M. Role of Fungi in Agriculture. In Biostimulants in Plant Science; IntechOpen: London, UK, 2020. [Google Scholar]
- Carrol, G.C. The biology of endophytism in plants with particular reference to woody perennials. In Microbiology of Phyllosphere; Cambridge University Press: London, UK, 1986; pp. 205–222. [Google Scholar]
- Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Islam, B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.-J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, e0208150. [Google Scholar] [CrossRef]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Rahman, H.; Tawab, A.; Ahmad, A.; Ayaz, S. Cinnamic acid as an inhibitor of growth, flavonoids exudation and endophytic fungus colonization in maize root. Plant Physiol. Biochem. 2019, 135, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef]
- Hussain, A.; Shah, S.T.; Rahman, H.; Irshad, M.; Iqbal, A. Effect of IAA on in vitro growth and colonization of Nostoc in plant roots. Front. Plant Sci. 2015, 6, 46. [Google Scholar] [CrossRef]
- Iqbal, A.A.; Farooq Shah, Y.J.; Hamayun, M.; Islam, B. Detection of food allergens by ELISA and other common methods. Fresenius Environ. Bull. 2018, 27, 8340–8346. [Google Scholar]
- Zuccaro, A.; Lahrmann, U.; Güldener, U.; Langen, G.; Pfiffi, S.; Biedenkopf, D.; Wong, P.; Samans, B.; Grimm, C.; Basiewicz, M. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 2011, 7, e1002290. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res. Int. 2018, 2018, 7696831. [Google Scholar]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Gabarrell, X.; Caminal, G.; Vicent, T.; Adinarayana Reddy, C. Aerobic degradation by white-rot fungi of trichloroethylene (TCE) and mixtures of TCE and perchloroethylene (PCE). J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 1190–1196. [Google Scholar] [CrossRef]
- Nikiforova, S.V.; Pozdnyakova, N.N.; Turkovskaya, O.V. Emulsifying agent production during PAHs degradation by the white rot fungus Pleurotus ostreatus D1. Curr. Microbiol. 2009, 58, 554–558. [Google Scholar] [CrossRef]
- Sivanesan, A. Graminicolous Species of Bipolaris, Curvularia, Drechslera, Exserohilum and Their Teleomorphs; CAB International: Wallingford, UK, 1987. [Google Scholar]
- Marin-Felix, Y.; Senwanna, C.; Cheewangkoon, R.; Crous, P.W. New species and records of Bipolaris and Curvularia from Thailand. Mycosphere 2017, 8, 1556–1574. [Google Scholar] [CrossRef]
- Hargreaves, M.; Parappukkaran, S.; Morawska, L.; Hitchins, J.; He, C.; Gilbert, D. A pilot investigation into associations between indoor airborne fungal and non-biological particle concentrations in residential houses in Brisbane, Australia. Sci. Total Environ. 2003, 312, 89–101. [Google Scholar] [CrossRef]
- Almaguer, M.; Rojas, T.I.; Rodríguez-Rajo, F.J.; Aira, M.J. Airborne fungal succession in a rice field of Cuba. Eur. J. Plant Pathol. 2012, 133, 473–482. [Google Scholar] [CrossRef]
- Strobel, G.; Kenfield, D.; Bunkers, G.; Sugawara, F.; Clardy, J. Phytotoxins as potential herbicides. Experientia 1991, 47, 819–826. [Google Scholar] [CrossRef]
- Tan, X.-M.; Zhou, Y.-Q.; Zhou, X.-L.; Xia, X.-H.; Wei, Y.; He, L.-L.; Tang, H.-Z.; Yu, L.-Y. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 2018, 8, 5929. [Google Scholar] [CrossRef]
- Khiralla, A.; Mohamed, I.E.; Tzanova, T.; Schohn, H.; Slezack-Deschaumes, S.; Hehn, A.; André, P.; Carre, G.; Spina, R.; Lobstein, A. Endophytic fungi associated with Sudanese medicinal plants show cytotoxic and antibiotic potential. FEMS Microbiol. Lett. 2016, 363, fnw089. [Google Scholar] [CrossRef]
- Khiralla, A.; Mohamed, I.; Thomas, J.; Mignard, B.; Spina, R.; Yagi, S.; Laurain-Mattar, D. A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants. Asian Pac. J. Trop. Med. 2015, 8, 701–704. [Google Scholar] [CrossRef]
- Madrid, H.; da Cunha, K.; Gené, J.; Dijksterhuis, J.; Cano, J.; Sutton, D.; Guarro, J.; Crous, P. Novel Curvularia species from clinical specimens. Pers.-Mol. Phylogeny Evol. Fungi 2014, 33, 48–60. [Google Scholar] [CrossRef]
- Manamgoda, D.S.; Rossman, A.; Castlebury, L.A.; Chukeatirote, E. Article PHYTOTAXA. Phytotaxa 2015, 212, 175–198. [Google Scholar] [CrossRef]
- Miqueleiz-Zapatero, A.; Santa Olalla-Peralta, C.; Guerrero-Torres, M.D.; Cardeñoso-Domingo, L.; Hernández-Milán, B.; Domingo-García, D. Mycobacterium lentiflavum as the main cause of lymphadenitis in pediatric population. Enferm. Infecc. Microbiol. Clin. Engl. Ed. 2018, 36, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Vasikasin, V.; Nasomsong, W.; Srisuttiyakorn, C.; Mitthamsiri, W.; Oer-Areemitr, N.; Changpradub, D. Disseminated phaeohyphomycosis caused by Curvularia tuberculata in a previously healthy man. Mycopathologia 2019, 184, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Evidente, A. Chloromonilinic acids C and D, phytotoxic tetrasubstituted 3-chromanonacrylic acids isolated from Cochliobolus australiensis with potential herbicidal activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 2771–2777. [Google Scholar] [CrossRef]
- He, J.; Yang, M.-S.; Wang, W.-X.; Li, Z.-H.; Elkhateeb, W.A.M.; Wen, T.-C.; Ai, H.-L.; Feng, T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131. [Google Scholar] [CrossRef]
- Boedijn, K.B. The genera Phillipsia and Cookeina in Netherlands India. Bull. Jard. Bot. Buitenzorg Ser. III 1933, 3, 57–76. [Google Scholar]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.-K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef]
- Campos, F.F.; Ramos, J.P.; de Oliveira, D.M.; Alves, T.; Souza-Fagundes, D.; Elaine, M.; Zani, C.L.; Sampaio, F.C.; Converti, A.; Cota, B.B. In vitro leishmanicidal, antibacterial and antitumour potential of anhydrocochlioquinone A obtained from the fungus Cochliobolus sp. J. Biosci. 2017, 42, 657–664. [Google Scholar] [CrossRef]
- Hyde, K.; Chomnunti, P.; Crous, P.; Groenewald, J.; Damm, U.; Ko, T.; Shivas, R.; Summerell, B.; Tan, Y. A case for re-inventory of Australia’s plant pathogens. Pers.-Mol. Phylogeny Evol. Fungi 2010, 25, 50–60. [Google Scholar] [CrossRef]
- Berbee, M.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Tan, Y.P.; Madrid, H.; Crous, P.W.; Shivas, R.G. Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas. Plant Pathol. 2014, 43, 589–603. [Google Scholar] [CrossRef]
- Deng, H.; Tan, Y.P.; Shivas, R.G.; Niu, Y.C. Curvularia tsudae comb. nov. et nom. nov., formerly Pseudocochliobolus australiensis, and a revised synonymy for Curvularia australiensis. Mycoscience 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Abdullahi, M.D.; Whong, C.M.; Sohunago, J.W.; Kassem, H.S.; Yaro, C.A.; Hetta, H.F.; Mostafa-Hedeab, G.; Zouganelis, G.D.; Batiha, G.E.-S. Characterization and phylogeny of fungi isolated from industrial wastewater using multiple genes. Sci. Rep. 2022, 12, 2094. [Google Scholar] [CrossRef]
- Shenoy, B.D.; Jeewon, R.; Wang, H.; Amandeep, K.; Ho, W.H.; Bhat, D.J.; Crous, P.W.; Hyde, K.D. Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Divers. 2010, 44, 161–169. [Google Scholar] [CrossRef]
- Hyde, K.; Cai, L.; Cannon, P.; Crouch, J.; Crous, P.; Damm, U.; Goodwin, P.; Chen, H.; Johnston, P.; Jones, E. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Cai, L.; Hyde, K.; Taylor, P.; Weir, B.; Waller, J.; Abang, M.; Zhang, J.; Yang, Y.; Phoulivong, S.; Liu, Z. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Hawksworth, D.L.; Crous, P.W.; Redhead, S.A.; Reynolds, D.R.; Samson, R.A.; Seifert, K.A.; Taylor, J.W.; Wingfield, M.J.; Abaci, Ö.; Aime, C. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2011, 2, 105–111. [Google Scholar] [CrossRef]
- Alcorn, J. On the genera Cochliobolus and Pseudocochliobolus [Fungi, genera distinction, conidium septation, new taxa]. Mycotaxon 1983, 16, 353–379. [Google Scholar]
- Manamgoda, D.S.; Cai, L.; Bahkali, A.H.; Chukeatirote, E.; Hyde, K.D. Cochliobolus: An overview and current status of species. Fungal Divers. 2011, 51, 3–42. [Google Scholar] [CrossRef]
- Hyde, K.; McKenzie, E.; KoKo, T. Towards incorporating anamorphic fungi in a natural classification–checklist and notes for 2010. Mycosphere 2011, 2, 1–88. [Google Scholar]
- Shoemaker, R. Nomenclature of Drechslera and Bipolaris, grass parasites segregated from ‘Helminthosporium’. Can. J. Bot. 1959, 37, 879–887. [Google Scholar] [CrossRef]
- Drechsler, C. Phytopathological and taxonomic aspects of Ophiobolus, Pyrenophora, Helminthosporium, and a new genus, Cochliobolus. Phytopathology 1934, 24, 953–985. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Egham, UK, 1971. [Google Scholar]
- Blakeman, J.P.; Fokkema, N.J. Potential for biological control of plant diseases on the phylloplane. Annu. Rev. Phytopathol. 1982, 20, 167–190. [Google Scholar] [CrossRef]
- Santamaría, J.; Bayman, P. Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microb. Ecol. 2005, 50, 1–8. [Google Scholar] [CrossRef]
- Alvindia, D.G.; Natsuaki, K.T. Evaluation of fungal epiphytes isolated from banana fruit surfaces for biocontrol of banana crown rot disease. Crop Prot. 2008, 27, 1200–1207. [Google Scholar] [CrossRef]
- Suryanarayanan, T.; Murali, T.; Venkatesan, G. Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can. J. Bot. 2002, 80, 818–826. [Google Scholar] [CrossRef]
- Thongkantha, S.; Lumyong, S.; McKenzie, E.; Hyde, K.D. Fungal saprobes and pathogens occurring on tissues of Dracaena lourieri and Pandanus spp. in Thailand. Fungal Divers. 2008, 30, 149–169. [Google Scholar]
- Larran, S.; Perelló, A.; Simón, M.R.; Moreno, V. The endophytic fungi from wheat (Triticum aestivum L.). World J. Microbiol. Biotechnol. 2007, 23, 565–572. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.; Imran, M.; Lee, K.; Kang, S.; Shin, J.; Joo, G.; Yun, B.; Lee, I. The Combined Inoculation of Curvularia lunata AR11 and Biochar Stimulates Synthetic Silicon and Potassium Phosphate Use Efficiency, and Mitigates Salt and Drought Stresses in Rice. Front. Plant Sci. 2022, 13, 816858. [Google Scholar] [CrossRef]
- Safi, A.; Mehrabi-Koushki, M.; Farokhinejad, R. Amesia khuzestanica and Curvularia iranica spp. nov. from Iran. Mycol. Prog. 2020, 19, 935–945. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; McKenzie, E.; Hyde, K. Are some endophytes of Musa acuminata latent pathogens? Fungal Divers. 2004, 16, 131–140. [Google Scholar]
- Fisher, P.; Petrini, O. Fungal saprobes and pathogens as endophytes of rice (Oryza sativa L.). New Phytol. 1992, 120, 137–143. [Google Scholar] [CrossRef]
- Morsy, M.R.; Oswald, J.; He, J.; Tang, Y.; Roossinck, M.J. Teasing apart a three-way symbiosis: Transcriptome analyses of Curvularia protuberata in response to viral infection and heat stress. Biochem. Biophys. Res. Commun. 2010, 401, 225–230. [Google Scholar] [CrossRef]
- Sakayaroj, J.; Preedanon, S.; Supaphon, O.; Jones, E.G.; Phongpaichit, S. Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Divers. 2010, 42, 27–45. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Zhu, P.; Yang, J.; Cheng, K. Phylogenetic diversity of culturable fungi associated with two marine sponges: Haliclona simulans and Gelliodes carnosa, collected from the Hainan Island coastal waters of the South China Sea. Fungal Divers. 2010, 42, 1–15. [Google Scholar] [CrossRef]
- Zakaria, L.; Aziz, W.N.W. Molecular identification of endophytic fungi from banana leaves (Musa spp.). Trop. Life Sci. Res. 2018, 29, 201. [Google Scholar] [CrossRef]
- Jajere, S.M.; Hassan, L.; Aziz, S.A.; Zakaria, Z.; Abu, J.; Nordin, F.; Faiz, N.M. Salmonella in native “village” chickens (Gallus domesticus): Prevalence and risk factors from farms in South-Central Peninsular Malaysia. Poult. Sci. 2019, 98, 5961–5970. [Google Scholar] [CrossRef]
- Basri, A.A.; Zuber, M.; Basri, E.I.; Zakaria, M.S.; Aziz, A.F.; Tamagawa, M.; Ahmad, K.A. Fluid structure interaction on paravalvular leakage of transcatheter aortic valve implantation related to aortic stenosis: A patient-specific case. Comput. Math. Methods Med. 2020, 2020, 9163085. [Google Scholar] [CrossRef] [PubMed]
- Larrán, S.; Mónaco, C. 12 Status and Progress of Research in Endophytes from Agricultural Crops in Argentina. Manag. Fungal Plant Pathog. 2010, 2010, 149. [Google Scholar]
- Surridge, A. Fungi associated with banana foliage in South Africa. Mycosphaerella Leaf Spot Dis. Banan. Present Status Outlook 2002, 2002, 99. [Google Scholar]
- Furtado, M. Micobiota Associada a Folhas de Bananeira em Cabo Verde; Instituto Superior de Agronomia: Lisbon, Portugal, 2011. [Google Scholar]
- Crous, P.W.; Petrini, O.; Marais, G.F.; Pretorius, Z.A.; Rehder, F. Occurrence of fungal endophytes in cultivars of Triticum aestivum in South Africa. Mycoscience 1995, 36, 105–111. [Google Scholar] [CrossRef]
- Campos, F.F.; Rosa, L.H.; Cota, B.B.; Caligiorne, R.B.; Teles Rabello, A.L.; Alves, T.M.A.; Rosa, C.A.; Zani, C.L. Leishmanicidal metabolites from Cochliobolus sp., an endophytic fungus isolated from Piptadenia adiantoides (Fabaceae). PLoS Negl. Trop. Dis. 2008, 2, e348. [Google Scholar] [CrossRef]
- Ataides, D.; Pamphile, J.A.; Garcia, A.; dos Santos Ribeiro, M.A.; Polonio, J.C.; Sarragiotto, M.H.; Clementeâ, E. Curvularin produced by endophytic Cochliobolus sp. G2-20 isolated from Sapindus saponaria L. and evaluation of biological activity. J. Appl. Pharm. Sci. 2018, 8, 032–037. [Google Scholar]
- Morell-Rodríguez, G. Potential of Fungal Endophytes from Thalassia testudinum Bank ex KD Koenig as Producers of Bioactive Compounds. Ph.D. Thesis, University of Puerto Rico, Mayaguez, Puerto Rico, 2008. [Google Scholar]
- Raviraja, N. Fungal endophytes in five medicinal plant species from Kudremukh Range, Western Ghats of India. J. Basic Microbiol. Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2005, 45, 230–235. [Google Scholar] [CrossRef]
- Raviraja, N.; Maria, G.; Sridhar, K. Antimicrobial evaluation of endophytic fungi inhabiting medicinal plants of the Western Ghats of India. Eng. Life Sci. 2006, 6, 515–520. [Google Scholar] [CrossRef]
- Herrero, N.; Sánchez Márquez, S.; Zabalgogeazcoa, I. Mycoviruses are common among different species of endophytic fungi of grasses. Arch. Virol. 2009, 154, 327–330. [Google Scholar] [CrossRef]
- Sánchez Márquez, S.; Bills, G.F.; Domínguez Acuña, L.; Zabalgogeazcoa, I. Endophytic mycobiota of leaves and roots of the grass Holcus lanatus. Fungal Divers. 2010, 41, 115–123. [Google Scholar] [CrossRef]
- Verma, V.C.; Kharwar, R.N. Efficacy of neem leaf extract against it’s own fungal endophyte Curvularia lunata. J. Agric. Technol. 2006, 2, 329–335. [Google Scholar]
- Suryanarayanan, T.; Venkatesan, G.; Murali, T.S. Endophytic fungal communities in leaves of tropical forest trees: Diversity and distribution patterns. Curr. Sci. 2003, 85, 89–493. [Google Scholar]
- Sukarno, N.; Ginting, R.C.B.; Widyastuti, U.; Darusman, L.K.; Kanaya, S.; Batubara, I.; Aryantha, I.N.P.; Waite, M. Endophytic Fungi from Four Indonesian Medicinal Plants and Their Inhibitory Effect on Plant Pathogenic Fusarium oxysporum. Hayati J. Biosci. 2021, 28, 152. [Google Scholar] [CrossRef]
- Asaf, S.; Jan, R.; Khan, M.A.; Khan, A.L.; Asif, S.; Bilal, S.; Ahmad, W.; Waqas, M.; Kim, K.-M.; Ahmed, A.-H. Unraveling the mutualistic interaction between endophytic Curvularia lunata CSL1 and tomato to mitigate cadmium (Cd) toxicity via transcriptomic insights. Sci. Total Environ. 2022, 861, 160542. [Google Scholar] [CrossRef]
- Ellis, M.B. Dematiaceous Hyphomycetes. VII: Curvularia, Brachy-Sporium etc.; Commonwealth Mycological Institute: Egham, UK, 1966. [Google Scholar]
- Kodsueb, R.; McKenzie, E.; Lumyong, S.; Hyde, K. Diversity of saprobic fungi on Magnoliaceae. Fungal Divers. 2008, 30, 37–53. [Google Scholar]
- Hyde, K.; Zhou, D.; McKenzie, E.; Ho, W.; Dalisay, T. Vertical distribution of saprobic fungi on bamboo culms. Fungal Divers. 2002, 11, 109–118. [Google Scholar]
- Mohamed, R.; Jong, P.; Zali, M. Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers. 2010, 43, 67–74. [Google Scholar] [CrossRef]
- Kobayashi, T. Index of Fungi Inhabiting Woody Plants in Japan; Zenkoku-Noson-Kyoiku Kyokai Publishing: Tokyo, Japan, 2007. [Google Scholar]
- Tokumasu, S.; Tubaki, K.; Manoch, L. A preliminary list of hyphomycetes isolated from pine leaf litter of Thailand. Rep. Tottori Mycol. Inst. 1990, 28, 185–190. [Google Scholar]
- Christensen, C.M.; Meronuck, R.A. Quality Maintenance in Stored Grains and Seeds; University of Minnesota Press: Minneapolis, MN, USA, 1986. [Google Scholar]
- Ebbels, D.; Allen, D. A Supplementary and Annotated List of Plant Diseases, Pathogens and Associated Fungi in Tanzania; Commonwealth Mycological Institute: Egham, UK, 1979. [Google Scholar]
- Liu, P.S.W. A supplement to a host list of plant diseases in Sabah, Malaysia. In A Supplement to a Host List of Plant Diseases in Sabah, Malaysia; Commonwealth Mycological Institute: Egham, UK, 1977. [Google Scholar]
- Peregrine, W.; Bin Ahmad, K. Grafting—A simple technique for overcoming bacterial wilt in tomato. Int. J. Pest Manag. 1982, 28, 71–76. [Google Scholar] [CrossRef]
- Grand, L. North Carolina Plant Disease Index, Issue 240 (Revised) of Technical Bulletin; North Carolina Agricultural Experiment Station: Raleigh, NC, USA, 1985. [Google Scholar]
- Cho, W.-D.; Shin, H.-D. List of Plant Diseases in Korea; Korean Society of Plant Pathology: Seoul, Republic of Korea, 2004. [Google Scholar]
- Hussain, M.A.; Mahajan, V.; Rather, I.A.; Awasthi, P.; Chouhan, R.; Dutt, P.; Sharma, Y.P.; Bedi, Y.S.; Gandhi, S.G. Isolation and identification of growth promoting endophytes from Artemisia annua L. and its effects on artemisinin content. Trends Phytochem. Res. 2017, 1, 207–214. [Google Scholar]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Ranf, S.; Drzewiecki, C.; Mithöfer, A.; Mazars, C.; Scheel, D.; Lee, J.; Oelmüller, R. A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J. 2009, 59, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.G. Chitin and Chitosan in Fungi; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lei, C.; Ma, D.; Pu, G.; Qiu, X.; Du, Z.; Wang, H.; Li, G.; Ye, H.; Liu, B. Foliar application of chitosan activates artemisinin biosynthesis in Artemisia annua L. Ind. Crops Prod. 2011, 33, 176–182. [Google Scholar] [CrossRef]
- Mondal, M.M.; Malek, M.; Puteh, A.; Ismail, M.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop Sci. 2012, 6, 918–921. [Google Scholar]
- Bibi, N.; Jan, G.; Jan, F.G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Rehman, H.; Tawab, A.; Khushdil, F. Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant Physiol. Biochem. 2019, 139, 459–469. [Google Scholar] [CrossRef]
- Bengyella, L.; Iftikhar, S.; Nawaz, K.; Fonmboh, D.J.; Yekwa, E.L.; Jones, R.C.; Njanu, Y.M.; Roy, P. Biotechnological application of endophytic filamentous bipolaris and curvularia: A review on bioeconomy impact. World J. Microbiol. Biotechnol. 2019, 35, 69. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Nukina, M.; Hattori, H.; Marumo, S. Cis-Sativenediol, a plant growth promotor, produced by fungi. J. Am. Chem. Soc. 1975, 97, 2542–2543. [Google Scholar] [CrossRef]
- Lubna; Khan, M.A.; Asaf, S.; Jan, R.; Waqas, M.; Kim, K.-M.; Lee, I.-J. Endophytic fungus Bipolaris sp. CSL-1 induces salt tolerance in Glycine max L. via modulating its endogenous hormones, antioxidative system and gene expression. J. Plant Interact. 2022, 17, 319–332. [Google Scholar] [CrossRef]
- Yousaf, M.J.; Hussain, A.; Hamayun, M.; Iqbal, A.; Irshad, M.; Kim, H.-Y.; Lee, I.-J. Transformation of endophytic Bipolaris spp. into biotrophic pathogen under auxin cross-talk with brassinosteroids and abscisic acid. Front. Bioeng. Biotechnol. 2021, 9, 657635. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–15. [Google Scholar]
- Ali, M.M.; Dipak, V. Potential of endophytic bacteria isolated from Vitex negundo L. to produce auxin. Res. J. Recent Sci. 2014, 3, 38–42. [Google Scholar]
- Ishida, Y.; Nakamura, A.; Mitani, Y.; Suzuki, M.; Soeno, K.; Asami, T.; Shimada, Y. Comparison of indole derivatives as potential intermediates of auxin biosynthesis in Arabidopsis. Plant Biotechnol. 2013, 30, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.; Khan, A.L.; Shahzad, R.; Ullah, I.; Khan, A.R.; Lee, I.-J. Mutualistic fungal endophytes produce phytohormones and organic acids that promote japonica rice plant growth under prolonged heat stress. J. Zhejiang Univ.-Sci. B 2015, 16, 1011–1018. [Google Scholar] [CrossRef]
- Hasan, H. Gibberellin and auxin production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Rostl. Vyrob. 2002, 48, 101–106. [Google Scholar]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Khan, A.L.; Kang, S.-M.; Dhakal, K.H.; Hussain, J.; Adnan, M.; Kim, J.-G.; Lee, I.-J. Flavonoids and amino acid regulation in Capsicum annuum L. by endophytic fungi under different heat stress regimes. Sci. Hortic. 2013, 155, 1–7. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Gunatilaka, M.K.; Wijeratne, K.; Gunatilaka, L.; Arnold, A.E. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS ONE 2013, 8, e73132. [Google Scholar] [CrossRef]
- Lubna; Asaf, S.; Khan, A.L.; Waqas, M.; Kang, S.-M.; Hamayun, M.; Lee, I.-J.; Hussain, A. Growth-promoting bioactivities of Bipolaris sp. CSL-1 isolated from Cannabis sativa suggest a distinctive role in modifying host plant phenotypic plasticity and functions. Acta Physiol. Plant. 2019, 41, 65. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Khan, A.L.; Kang, S.M.; Lee, I.-J. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann. Microbiol. 2015, 65, 585–593. [Google Scholar] [CrossRef]
- Mandyam, K.; Loughin, T.; Jumpponen, A. Isolation and morphological and metabolic characterization of common endophytes in annually burned tallgrass prairie. Mycologia 2010, 102, 813–821. [Google Scholar] [CrossRef]
- Mahamuni, S.; Wani, P.; Patil, A. Isolation of phosphate solubilizing fungi from rhizosphere of sugarcane & sugar beet using TCP & RP solubilization. Asian J. Biochem. Pharm. Res. 2012, 2, 237–244. [Google Scholar]
- Kapri, A.; Tewari, L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010, 41, 787–795. [Google Scholar] [CrossRef]
- Pradhan, N.; Sukla, L. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr. J. Biotechnol. 2006, 5, 850–854. [Google Scholar]
- Gupta, N.; Sabat, J.; Parida, R.; Kerkatta, D. Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot. Croat. 2007, 66, 197–204. [Google Scholar]
- Lubna; Asaf, S.; Hamayun, M.; Gul, H.; Lee, I.-J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Walton, J. Regulation of cyclic peptide biosynthesis and pathogenicity in Cochliobolus carbonum by TOXEp, a novel protein with a bZIP basic DNA-binding motif and four ankyrin repeats. Mol. Gen. Genet. MGG 1998, 260, 462–469. [Google Scholar] [CrossRef]
- Nakajima, H.; Fujimoto, H.; Matsumoto, R.; Hamasaki, T. Biosynthesis of spiciferone A and spicifernin, bioactive metabolites of the phytopathogenic fungus, Cochliobolus spicifer. J. Org. Chem. 1993, 58, 4526–4528. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Rowland, C.Y. 6-Chlorodehydrocurvularin, a new metabolite from Cochliobolus spicifer. J. Nat. Prod. 1993, 56, 2175–2177. [Google Scholar] [CrossRef]
- Carruthers, J.; Cerrini, S.; Fedeli, W.; Casinovi, C.; Galeffi, C.; Vaccaro, A.T.; Scala, A. Structures of cochlioquinones A and B, new metabolites of Cochliobolus miyabeanus: Chemical and X-ray crystallographic determination. J. Chem. Soc. D Chem. Commun. 1971, 3, 164–166. [Google Scholar] [CrossRef]
- Raghunath, R.; Radhakrishna, A.; Angayarkanni, J.; Palaniswamy, M. Production and cytotoxicity studies of lovastatin from aspergillus niger PN2 an endophytic fungi isolated from Taxus baccata. Int. J. Appl. Biol. Pharm. Technol. 2012, 3, 342–351. [Google Scholar]
- Han, W.B.; Lu, Y.H.; Zhang, A.H.; Zhang, G.F.; Mei, Y.N.; Jiang, N.; Lei, X.; Song, Y.C.; Ng, S.W.; Tan, R.X. Curvulamine, a new antibacterial alkaloid incorporating two undescribed units from a Curvularia species. Org. Lett. 2014, 16, 5366–5369. [Google Scholar] [CrossRef]
- Wells, J.M.; Cole, R.; Cutler, H.; Spalding, D. Curvularia lunata, a new source of cytochalasin B. Appl. Environ. Microbiol. 1981, 41, 967–971. [Google Scholar] [CrossRef]
- Abraham, W.-R.; Meyer, H.; Abate, D. Curvupallides, a new class of alkaloids from the fungus Curvularia pallescens. Tetrahedron 1995, 51, 4947–4952. [Google Scholar] [CrossRef]
- Sugawara, F.; Strobel, G.; Fisher, L.; van Duyne, G.; Clardy, J. Bipolaroxin, a selective phytotoxin produced by Bipolaris cynodontis. Proc. Natl. Acad. Sci. USA 1985, 82, 8291–8294. [Google Scholar] [CrossRef]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; Kelter, G.; Maier, A.; Fiebig, H.H.; König, G.M. Apralactone A and a new stereochemical class of curvularins from the marine fungus Curvularia sp. Eur. J. Org. Chem. 2008, 30, 5085–5092. [Google Scholar] [CrossRef]
- Chomcheon, P.; Wiyakrutta, S.; Aree, T.; Sriubolmas, N.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Curvularides A–E: Antifungal hybrid peptide–polyketides from the endophytic fungus Curvularia geniculata. Chem.-Eur. J. 2010, 16, 11178–11185. [Google Scholar] [CrossRef]
- Samanthi, K.; Wickramarachchi, S.; Wijeratne, E.; Paranagama, P. Two new bioactive polyketides from Curvularia trifolii, an endolichenic fungus isolated from Usnea sp., in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2015, 43, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.; Khan, M.A.; Qureshi, A.A. Studies in the biochemistry of micro-organisms—II: Constitution of curvulin, curvulinic acid and curvulol, metabolic products of curvularia siddiqui. Tetrahedron 1963, 19, 111–115. [Google Scholar] [CrossRef]
- Busi, S.; Peddikotla, P.; Upadyayula, S.M.; Yenamandra, V. Secondary metabolites of Curvularia oryzae MTCC 2605. Rec. Nat. Prod. 2009, 3, 204. [Google Scholar]
- Arunpanichlert, J.; Rukachaisirikul, V.; Tadpetch, K.; Phongpaichit, S.; Hutadilok-Towatana, N.; Supaphon, O.; Sakayaroj, J. A dimeric chromanone and a phthalide: Metabolites from the seagrass-derived fungus Bipolaris sp. PSU-ES64. Phytochem. Lett. 2012, 5, 604–608. [Google Scholar] [CrossRef]
- Baker, S.E.; Kroken, S.; Inderbitzin, P.; Asvarak, T.; Li, B.-Y.; Shi, L.; Yoder, O.C.; Turgeon, B.G. Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol. Plant-Microbe Interact. 2006, 19, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, S.; Wang, L.; Hou, E.; Xiao, D. First report of leaf blight of rice caused by Cochliobolus lunatus in China. Plant Dis. 2014, 98, 686. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.; Brooks, R.; Howes, A.; Kirkman, J.; Gregg, P. The potential of the high-biomass nickel hyperaccumulator Berkheya coddii for phytoremediation and phytomining. J. Geochem. Explor. 1997, 60, 115–126. [Google Scholar] [CrossRef]
- Nukina, M. Radicinol, a New metabolite of Cochliobolus lunata, and absolute stereochemistry of radicinin. Tetrahedron Lett. 1977, 18, 3271–3272. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce’s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef]
- Nukina, M.; Marumo, S. α-Acetylorcinol from Cochliobolus lunata. Agric. Biol. Chem. 1977, 41, 717. [Google Scholar] [CrossRef]
- Van Eijk, G.; Roeymans, H. Cynodontin, the tetrahydroxyanthraquinone of Curvularia and Drechslera species. Experientia 1977, 33, 1283–1284. [Google Scholar] [CrossRef]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono, A.; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef]
- Steglich, W.; Fugmann, B.; Lang-Fugmann, S. Römpp Encyclopedia Natural Products; Georg Thieme Verlag: Stuttgart, Germany, 2000. [Google Scholar]
- Bills, G.F.; Peláez, F.; Polishook, J.D.; Diez-Matas, M.T.; Harris, G.H.; Clapp, W.H.; Dufresne, C.; Byrne, K.M.; Nallin-Omstead, M.; Jenkins, R.G. Distribution of zaragozic acids (squalestatins) among filamentous ascomycetes. Mycol. Res. 1994, 98, 733–739. [Google Scholar] [CrossRef]
- Munro, H.D.; Musgrave, O.C.; Turner, A.B. 3α-Hydroxy-5β-chol-11-en-24-oic acid, a new fungal metabolite. J. Chem. Soc. Perkin Trans. 1974, 1, 1597–1598. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Sawang, K.; Siripong, P.; Tip-Pyang, S. Anhydrocochlioquinone A, a new antitumor compound from Bipolaris oryzae. Tetrahedron Lett. 2007, 48, 5193–5195. [Google Scholar] [CrossRef]
- Li, E.; Clark, A.M.; Rotella, D.P.; Hufford, C.D. Microbial metabolites of ophiobolin A and antimicrobial evaluation of ophiobolins. J. Nat. Prod. 1995, 58, 74–81. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Yang, J.-L.; Qi, Q.-Y.; Bao, L.; Yang, X.-L.; Liu, M.-M.; Huang, P.; Zhang, L.-X.; Chen, J.-L.; Cai, L. 3-Anhydro-6-hydroxy-ophiobolin A, a new sesterterpene inhibiting the growth of methicillin-resistant Staphylococcus aureus and inducing the cell death by apoptosis on K562, from the phytopathogenic fungus Bipolaris oryzae. Bioorganic Med. Chem. Lett. 2013, 23, 3547–3550. [Google Scholar] [CrossRef]
- Nukina, M.; Marumo, S. Aversion Factors, Antibiotics among Different Strains of a Fungal Species Aversion Factors of Cochliobolus setariae. Agric. Biol. Chem. 1976, 40, 2121–2123. [Google Scholar] [CrossRef]
- Wang, H.; Wei, S.; Yang, X. First report of Bipolaris leaf spot caused by Bipolaris oryzae on Typha orientalis in China. Plant Dis. 2019, 103, 1031. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, H.B.; Lim, C.-H.; Kim, C.-J.; Kwon, H.J. Cochlioquinone A1, a new anti-angiogenic agent from Bipolaris zeicola. Bioorganic Med. Chem. 2003, 11, 4743–4747. [Google Scholar] [CrossRef]
- Aldridge, D.; Turner, W. 9-Hydroxyprehelminthosporol, a metabolite of Cochliobolus (Helminthosporium) sativus. J. Chem. Soc. C Org. 1970, 5, 686–688. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Kolaibe, A.; Daba, G.M. Cochliobolus, Drechslera, Bipolaris, Curvularia different nomenclature for one potent fungus. J. Pharm. Pharmacol. Res. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Handayani, D.; Putri, R.; Ismed, F.; Hertiani, T.; Ariantari, N.; Proksch, P. Bioactive metabolite from marine sponge-derived fungus Cochliobolus geniculatus WR12. Rasayan J. Chem. 2020, 13, 417–422. [Google Scholar] [CrossRef]
- Avinash, K.; Ashwini, H.; Babu, H.; Krishnamurthy, Y. Antimicrobial potential of crude extract of Curvularia lunata, an endophytic fungi isolated from Cymbopogon caesius. J. Mycol. 2015, 2015, 185821. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Cheng, Y.-L.; Cai, C.-J.; Fan, L.; Gao, J.; Hou, C.-L. Diversity and antimicrobial activity of culturable endophytic fungi isolated from moso bamboo seeds. PLoS ONE 2014, 9, e95838. [Google Scholar] [CrossRef] [PubMed]
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol. Med. Microbiol. 2007, 51, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Geetha, V.; Venkatachalam, A.; Suryanarayanan, T.S.; Doble, M. Isolation and characterization of new antioxidant and antibacterial compounds from algicolous marine fungus Curvularia tuberculata. In Proceedings of the International Conference on Bioscience, Biochemistry and Bioinformatics (IPCBEE), Sentaosa, Singapore, 26–28 February 2011; pp. 16–18. [Google Scholar]
- Silva, M.; Almeida, A.; Arruda, F.; Gusmao, N. Endophytic fungi from brazilian mangrove plant Laguncularia racemosa (L.) Gaertn.(Combretaceae): Their antimicrobial potential. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 2, 1260–1266. [Google Scholar]
- Rekha, D.; Shivanna, M. Diversity, antimicrobial and antioxidant activities of fungal endophytes in Cynodon dactylon (L.) Pers. and Dactyloctenium aegyptium (L.) P. Beauv. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 573–591. [Google Scholar]
- Asensio, M.A.; Núñez, F.; Delgado, J.; Bermúdez, E. Control of toxigenic molds in food processing. In Microbial Food Safety and Preservation Techniques; Rai, V.R., Bai, A.J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 329–357. [Google Scholar]
- Ramesha, A.; Srinivas, C. Antimicrobial activity and phytochemical analysis of crude extracts of endophytic fungi isolated from Plumeria acuminata L. and Plumeria obtusifolia L. Eur. J. Exp. Biol. 2014, 4, 35–43. [Google Scholar]
- Juniper, S.; Abbott, L. Vesicular-arbuscular mycorrhizas and soil salinity. Mycorrhiza 1993, 4, 45–57. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Bengyella, L.; Yekwa, E.L.; Nawaz, K.; Iftikhar, S.; Tambo, E.; Alisoltani, A.; Feto, N.A.; Roy, P. Global invasive Cochliobolus species: Cohort of destroyers with implications in food losses and insecurity in the twenty-first century. Arch. Microbiol. 2018, 200, 119–135. [Google Scholar] [CrossRef]
- Calvert, C.; Smallwood, M.; Bowles, D.J. Plant Responses to Environmental Stress; Bios Scientific: The Woodlands, TX, USA, 1999. [Google Scholar]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Zhou, W.-N.; White, J.F.; Soares, M.A.; Torres, M.S.; Zhou, Z.-P.; Li, H.-Y. Diversity of fungi associated with plants growing in geothermal ecosystems and evaluation of their capacities to enhance thermotolerance of host plants. J. Plant Interact. 2015, 10, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Bengyella, L.; Roy, P.; Sayanika, D.W.; Narayan, C.; Ra, T. Report of foliar necrosis of potato caused by Cochliobolus lunatus in India. Afr. J. Biotechnol. 2013, 12, 833–835. [Google Scholar]
- Davidson, J.F.; Whyte, B.; Bissinger, P.H.; Schiestl, R.H. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1996, 93, 5116–5121. [Google Scholar] [CrossRef]
- Butler, M.; Day, A. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136. [Google Scholar] [CrossRef]
- Sangamesh, M.; Jambagi, S.; Vasanthakumari, M.; Shetty, N.J.; Kolte, H.; Ravikanth, G.; Nataraja, K.N.; Uma Shaanker, R. Thermotolerance of fungal endophytes isolated from plants adapted to the Thar Desert, India. Symbiosis 2018, 75, 135–147. [Google Scholar] [CrossRef]
- Yin, C.; Jin, L.; Sun, F.; Xu, X.; Shao, M.; Zhang, Y. Phytotoxic and Antifungal Metabolites from Curvularia crepinii QTYC-1 Isolated from the Gut of Pantala flavescens. Molecules 2018, 23, 951. [Google Scholar] [CrossRef]
- Khiralla, A.; Spina, R.; Saliba, S.; Laurain-Mattar, D. Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biol. Rev. 2019, 33, 101–122. [Google Scholar] [CrossRef]
- Butt, T.M.; Jackson, C.; Magan, N. Fungi as Biocontrol Agents: Progress Problems and Potential; CABI: Wallingford, UK, 2001. [Google Scholar]
- Evans, H.C.; Greaves, M.P.; Watson, A.K. Fungal biocontrol agents of weeds. In Fungi as Biocontrol Agents; CABI Publishing: Wallingford, UK, 2001; pp. 169–192. [Google Scholar]
- Casonato, S.; Lawrie, A.; McLaren, D. Prospects for biological control of serrated tussock. In Proceedings of the Tussock Terminators Research Forum, Albury, NSW, Australia, 2–3 November 2005; pp. 32–35. [Google Scholar]
- Oerke, E.-C.; Dehne, H.-W.; Schönbeck, F.; Weber, A. Crop Production and Crop Protection: Estimated Losses in Major Food and Cash Crops; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Gadermaier, G.; Hauser, M.; Ferreira, F. Allergens of weed pollen: An overview on recombinant and natural molecules. Methods 2014, 66, 55–66. [Google Scholar] [CrossRef]
- Hetherington, S.; Irwin, J. Pathological and molecular genetic variation in the interaction between Sporobolus spp. and Bipolaris spp. Aust. J. Agric. Res. 1999, 50, 583–588. [Google Scholar] [CrossRef]
- Figliola, S.S.; Camper, N.; Ridings, W. Potential biological control agents for goosegrass (Eleusine indica). Weed Sci. 1988, 36, 830–835. [Google Scholar] [CrossRef]
- Kleczewski, N.M.; Flory, S.L. Leaf blight disease on the invasive grass Microstegium vimineum caused by a Bipolaris sp. Plant Dis. 2010, 94, 807–811. [Google Scholar] [CrossRef]
- Jiang, S.J.; Qiang, S.; Zhu, Y.Z.; Dong, Y.F. Isolation and phytotoxicity of a metabolite from Curvularia eragrostidis and characterisation of its modes of action. Ann. Appl. Biol. 2008, 152, 103–111. [Google Scholar] [CrossRef]
- Motlagh, M.R.S. Evaluation of Curvularia lunata as an biological control agent in major weeds of rice paddies. Life Sci. J. 2011, 8, 81–91. [Google Scholar]
- Tsukamoto, H.; Gohbara, M.; Tsuda, M.; Fujimori, T. Evaluation of fungal pathogens as biological control agents for the paddy weed, Echinochloa species by drop inoculation. Jpn. J. Phytopathol. 1997, 63, 366–372. [Google Scholar] [CrossRef]
- De Luna, L.Z.; Watson, A.K.; Paulitz, T.C. Reaction of rice (Oryza sativa) cultivars to penetration and infection by Curvularia tuberculata and C. oryzae. Plant Dis. 2002, 86, 470–476. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, W.; Cao, H.; Feng, H. Mikania micrantha HBK in China—An overview. Weed Res. 2004, 44, 42–49. [Google Scholar] [CrossRef]
- Shujun, J.; Sheng, Q.; Yunzhi, Z. Isolation, purification, identification, and bioassay of helminthosporin with herbicidal activity from Curvularia eragrostidis. Acta Phytophylacica Sin. 2006, 33, 313–318. [Google Scholar]
- Adhikary, S.; Mian, I. GEL electrophoresis of extracellular enzymes of Bipolaris sorokiniana. Indian J. Agric. Res. 2006, 40, 235. [Google Scholar]
- Mohamed, I.A.; Neveen, M.K.; Mohamed, N.A.E.-G. Biodegradation of some polycyclic aromatic hydrocarbons by Aspergillus terreus. Afr. J. Microbiol. Res. 2012, 6, 3783–3790. [Google Scholar] [CrossRef]
- Diss, L.; Blaudez, D.; Gelhaye, E.; Chalot, M. Genome-wide analysis of fungal manganese transporters, with an emphasis on Phanerochaete chrysosporium. Environ. Microbiol. Rep. 2011, 3, 367–382. [Google Scholar] [CrossRef]
- Sumathi, T.; Viswanath, B.; Sri Lakshmi, A.; SaiGopal, D. Production of laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem. Res. Int. 2016, 2016, 9519527. [Google Scholar] [CrossRef]
- Vázquez, M.A.; Cabrera, E.C.V.; Aceves, M.A.; Mallol, J.L.F. Cellulolytic and ligninolytic potential of new strains of fungi for the conversion of fibrous substrates. Biotechnol. Res. Innov. 2019, 3, 177–186. [Google Scholar] [CrossRef]
- Pádua, R.M.; Oliveira, A.B.; Souza Filho, J.D.; Takahashi, J.A.; Silva, M.d.A.; Braga, F.C. Biotransformation of digitoxigenin by Cochliobolus lunatus. J. Braz. Chem. Soc. 2007, 18, 1303–1310. [Google Scholar] [CrossRef]
- Abu-Elreesh, G.; Zaki, S.; Farag, S.; Elkady, M.F.; Abd-El-Haleem, D. Exobiopolymer from polyhydroxyalkanoate-producing transgenic yeast. Afr. J. Biotechnol. 2011, 10, 6558–6563. [Google Scholar]
- Flowers, T.; Pulford, I.; Duncan, H. Studies on the breakdown of oil in soil. Environ. Pollut. Ser. B Chem. Phys. 1984, 8, 71–82. [Google Scholar] [CrossRef]
- Neoh, C.H.; Lam, C.Y.; Yahya, A.; Ware, I.; Ibrahim, Z. Utilization of agro-industrial residues from palm oil industry for production of lignocellulolytic enzymes by Curvularia clavata. Waste Biomass Valorization 2015, 6, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Al-Nasrawi, H. Biodegradation of crude oil by fungi isolated from Gulf of Mexico. J. Bioremed. Biodegrad. 2012, 3, 147–152. [Google Scholar]
- Bhatt, J.K.; Ghevariya, C.M.; Dudhagara, D.R.; Rajpara, R.K.; Dave, B.P. Application of response surface methodology for rapid chrysene biodegradation by newly isolated marine-derived fungus Cochliobolus lunatus strain CHR4D. J. Microbiol. 2014, 52, 908–917. [Google Scholar] [CrossRef]
- Hadibarata, T.; Kristanti, R.A. Fate and cometabolic degradation of benzo [a] pyrene by white-rot fungus Armillaria sp. F022. Bioresour. Technol. 2012, 107, 314–318. [Google Scholar] [CrossRef]
- Bi, Y.; Yu, Z. Diterpenoids from Streptomyces sp. SN194 and their antifungal activity against Botrytis cinerea. J. Agric. Food Chem. 2016, 64, 8525–8529. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, N.; Kar, R.; Sukla, L.; Mishra, B. Microbial recovery of uranium using native fungal strains. Hydrometallurgy 2009, 95, 175–177. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Tanfani, F.; Scarpini, G. Chelating, film-forming, and coagulating ability of the chitosan–glucan complex from Aspergillus niger industrial wastes. Biotechnol. Bioeng. 1980, 22, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Kabran, P.; Rossignol, T.; Gaillardin, C.; Nicaud, J.-M.; Neuvéglise, C. Alternative Splicing Regulates Targeting of Malate Dehydrogenase in Yarrowia lipolytica. DNA Res. 2012, 19, 231–244. [Google Scholar] [CrossRef]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M. Life with 6000 genes. Science 1996, 274, 546–567. [Google Scholar] [CrossRef]
- Gao, S.; Li, Y.; Gao, J.; Suo, Y.; Fu, K.; Li, Y.; Chen, J. Genome sequence and virulence variation-related transcriptome profiles of Curvularia lunata, an important maize pathogenic fungus. BMC Genom. 2014, 15, 627. [Google Scholar] [CrossRef]
- Kuan, C.S.; Yew, S.M.; Toh, Y.F.; Chan, C.L.; Ngeow, Y.F.; Lee, K.W.; Na, S.L.; Yee, W.-Y.; Hoh, C.-C.; Ng, K.P. Dissecting the fungal biology of Bipolaris papendorfii: From phylogenetic to comparative genomic analysis. DNA Res. 2015, 22, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Zaccaron, A.Z.; Bluhm, B.H. The genome sequence of Bipolaris cookei reveals mechanisms of pathogenesis underlying target leaf spot of sorghum. Sci. Rep. 2017, 7, 17217. [Google Scholar] [CrossRef] [Green Version]


| Species | Accession Number | Host | Plant Part | References |
|---|---|---|---|---|
| Cochliobolus intermedius | Banana (Musa spp.) | Leaves | [63] | |
| Bipolaris papendorfii | Banana (Musa spp.) | Leaves | [64] | |
| Bipolaris sp. | Banana (Musa spp.) | Leaves | [65] | |
| Bipolaris cyanodontis | Solanum lycopersicum | leaves | [66] | |
| Bipolaris cyanodontis | Musa sp. | leaves | [66] | |
| Bipolaris sorokiniana | Triticum aestivum | leaves, glumes | [55,66] | |
| Bipolaris sp. | Triticum aestivum | Leaves, stem | [55] | |
| Cochliobolus australiensis | Musa sp. | Leaves | [67,68] | |
| Cochliobolus sativus | Triticum aestivum | Leaves | [69] | |
| Cochliobolus sp. (UFMGCB-555) | EU684269 | Piptadenia adiantoides | Leaves | [70] |
| Cochliobolus sp.(G2-20) | GQ461566.1 | Hevea brasiliensis | Leaves | [71] |
| Curvularia affinis | KJ909780 | Elaeodendron glaucum | Leaves | [53] |
| Curvularia brachyspora | Thalassia testudinum | Leaves | [72] | |
| Curvularia clavata | Adhatoda zeylanica | Leaves | [73] | |
| Curvularia clavata | Bauhinia phoenicea | Bark, leaves | [73] | |
| Curvularia clavata | Callicarpa tomentos | Bark, leaves | [74] | |
| Curvularia geniculata | Bauhinia phoenicea | Bark, leaves | [73] | |
| Curvularia inaequalis | Holcus lanatus | Leaves | [75,76] | |
| Curvularia lunatus | Callicarpa tomentosa | Bark, leaves | [73] | |
| Curvularia lunatus | Bauhinia phoenicea | Bark, leaves | [73] | |
| Curvularia lunatus | Callicarpa tomentosa | Leaves | [73] | |
| Curvularia lunatus | Ficus benghalensis | Leaves | [53] | |
| Curvularia lunatus | Azadirachta indica | Leaves | [77] | |
| Curvularia lunatus | Thalassia testudinum | Leaves | [72] | |
| Curvularia lunatus | Triticum aestivum | Leaves, glumes, grains | [55] | |
| Curvularia pallescens | Callicarpa tomentosa | Stem, leaves | [73] | |
| Cochliobolus spicifer | Triticum aesitivum | Grains | [55] | |
| Curvularia oryzae | Erythroxylon monogynum | Leaves | [53] | |
| Curvularia tuberculata (516) | (AC) 10336 | Elaeodendron glaucum, Dalbergia latifolia | Leaves | [78] |
| C. asiatica | JX256424 | Panicum sp. | Leaves | [79] |
| C. beerburrumensis | MH414894 | Eragrostis bahiensis | Leaves | [79] |
| C. cactivora | MN688803 | Member of Cactaceae | Leaves | [79] |
| C. chiangmaiensis | MF490814 | Zea mays | Leaves | [79] |
| C. coicis | JN192373 | Coix lacryma | Leaves | [79] |
| C. cymbopogonis | HG778985 | Yucca sp. | Leaves | [79] |
| C. dactyloctenii | KJ415545 | Dactyloctenium radulans | Leaves | [79] |
| C. eragrosticola | MH414899 | Eragrostis pilosa | Leaves | [79] |
| C. gladioli | HG778987 | Gladiolus sp. | Leaves | [79] |
| C. heteropogonis | JN192379 | Heteropogon contortus | Leaves | [79] |
| C. intermedia | HG778991 | Avena versicolor | Leaves | [79] |
| C. ischaemi | JX256428 | Ischaemum indicum | Leaves | [79] |
| C. microspore | MF139088 | Hippeastrum striatum | Leaf | [79] |
| C. mosaddeghii | MG846737 | Syzygium cumini | Leaf | [79] |
| C. neergaardii | KJ415550 | Oryza sativa | Leaves | [79] |
| C. nodosa | MF490816 | Digitaria ciliaris | Leaves | [79] |
| C. nodulosa | JN192383 | Eleusine indica | Leaves | [79] |
| C. oryzae | KP400650 | Oryza sativa | Leaves | [79] |
| C. ovariicola | MN688809 | Eragrostis interrupta | Leaves | [79] |
| C. palmicola | MF621582 | Acoelorrhaphe wrightii | Leaves | [79] |
| C. papendorfii | KJ909774 | Acacia karroo | Leaves | [79] |
| C. paterae | MN688810 | Triticum durum | Seed | [79] |
| C. perotidis | JN192385 | Perotis rara | Leaves | [79] |
| C. petersonii | MH414905 | Dactyloctenium aegyptium | Leaves | [79] |
| C. portulacae | KJ415553 | Portulaca oleracea | Leaves | [79] |
| C. sorghina | KJ415558 | Sorghum bicolor | Leaves | [79] |
| C. sporobolicola | MH414908 | Sporobolus australasicus | Leaves | [79] |
| C. thailandica | MH275057 | Pandanus sp. | Leaves | [79] |
| C. trifolii | HG779023 | Trifolium repens | Leaves | [79] |
| C. tuberculate | JX256433 | Zea mays | Leaves | [79] |
| C. xishuangbannaensis | MH275058 | Pandanus sp. | Leaves | [79] |
| Bipolaris maydis | KJ909769 | Zea mays | Leaves | [79] |
| B. sorokiniana | KJ922381 | Hordeum sp. | Leaves | [79] |
| Curvularia affinis | HG778981.1 | G. ulmifolia | Leaves | [79] |
| Curvularia lunata | OP265394 | Solanaum nigram | Root | [80] |
| Species | Host | Plant Part | References |
|---|---|---|---|
| Cochliobolus sasae | Sasa tambaensis (Poaceae) | Dead leaves | [85] |
| Bipolaris ellisii(ZG-9) | Pinus khasya | Dead leaves | [86] |
| Bipolaris maydis | Zea mays | Seeds | [87] |
| Curvularia cymbopogonis | Pinus caribaea | Damping-off of seedlings | [88] |
| Curvularia eragrostidis | Pinus caribaea | Root rot | [89] |
| Curvularia eragrostidis | Pinus khasya | Decaying and fallen needles | [86] |
| Curvularia eragrostidis | Phaseolus vulgaris | Pod rot | [90] |
| Curvularia eragrostidis | Pandanus | Decaying leaves | [54] |
| Curvularia eragrostidis | Pandanus odoratissimus | Decaying leaves | [54] |
| Cochliobolus geniculatus | Phaseolus vulgaris | Rotting pods | [90] |
| Curvularia intermedia | Zea mays subsp. Mays | Dead leaves | [91] |
| Curvularia intermedia | Zea mays | Dead leaves | [92] |
| Curvularia lunta | Bambusa sp. | Dead clumps | [83] |
| Curvularia pallescens | Phaseolus vulgaris | Pod rot | [90] |
| Curvularia pallescens | Pinus taiwanensis | Root rot | [89] |
| Curvularia tuberculatus | Pinus caribaea | From damped-off seedlings | [88] |
| Curvularia verruculosus | Pinus caribaea | From damped-off seedlings | [88] |
| Curvularia sp. | Magnolia liliifera | Woody litter | [82] |
| Cochliobolus sitharamii | Bambusa sp. (Poaceae) | Leaves | [45] |
| Species | Accession Number | References |
|---|---|---|
| Cochliobolus lunatus | MK611790 | [99] |
| Bipolaris sp. | [100] | |
| C. geniculata | KX656363 | [100,101] |
| Curvularia pallescens | KJ534376 | [93] |
| Cochliobolus lunatus | KJ534378 | [93] |
| Cochliobolus setariae IFO 6635 | [102] | |
| Curvularia lunata AR11 | MZ145250 | [56] |
| Bipolaris sp. CSL-1 | [103] | |
| Bipolaris spp. | [104] |
| Chemical Classes | Natural Products | Biological Activities | Fungi | References |
|---|---|---|---|---|
| Alkaloids | curvulamine | antimicrobial, anti-inflammatory | Curvularia sp. IFBZ10 | [126] |
| curindolizine | anti-inflammatory | Curvularia sp. IFBZ10 | [126] | |
| cytochalasin B | Curvularia lunata | [127] | ||
| curvupallide A | Curvularia pallescens | [128] | ||
| curvupallide B | ||||
| curvupallide C | ||||
| spirostaphylotrichin A | ||||
| spirostaphylotrichin C | ||||
| spirostaphylotrichin D | ||||
| spirostaphylotrichin Q | ||||
| spirostaphylotrichin R | ||||
| spirostaphylotrichin U | ||||
| spirostaphylotrichin V | ||||
| bipolaramide | Bipolaris sorokiniana | [129] | ||
| Peptides | victorin C | phytotoxicity | Bipolaris victoriae | [100] |
| HC-toxin | Bipolaris arbonum | [100] | ||
| Polyketides | apralactone A | cytotoxicity | Curvularia sp. | [130] |
| (þ)-(15R)-10,11-E-dehydrocurvularin | ||||
| (þ)-(15R)-12-hydroxy-10,11-E-dehydrocurvularin | ||||
| (þ)-(11R,15R)-11-hydroxycurvularin | ||||
| (þ)-(15R)-12-oxocurvularin | ||||
| curvularin | natural biopesticides, antimicrobial | Cochliobolus sp. | [71] | |
| curvularide A, C, D, E | Curvularia geniculata | |||
| curvularide B | antifungal property | Curvularia geniculata | [131] | |
| curvulide A | ||||
| curvulide B1 | ||||
| curvulide B2 | ||||
| 6-chlorodehydrocurvularin | leishmanicidal activity | Cochliobolus spicifer | [70] | |
| modiolide A | ||||
| 1,14-dihydroxy-6-methyl-6,7,8,9,10,10a,14,14a-octahydro-1H-benzo[f][1]oxacyclododecin-4(13H)-one. | antioxidant, anticancer | Curvularia trifolii. US/US/06 | [132] | |
| 5-methoxy-4,8,15-tri methyl-3,7-dioxo-1,3,7,8,9,10,11,12,13,14,15 | anti-inflammatory, antioxidant | |||
| 15a dodecahydrocyclododeca [de] isochromene -15-carboxylic acid | ||||
| curvulinic acid | Curvularia siddiqui | [133] | ||
| curvulol | ||||
| methyl2-acetyl-3,5 dihydroxyphenylacetate | Curvularia siddiqui | |||
| methyl2-acetyl-5-hydroxy-3-methoxyphenylacetate | Curvularia lunata | |||
| curvulin | ||||
| 11-a-methoxycurvularin | antibacterial antifungal | Curvularia oryzae | [134] | |
| (S)-5-ethyl-8, 8-dimethylnonanal | antilarval | |||
| bipolarinone | Bipolaris sp. PSU-ES64 | [135] | ||
| bipolarilide | ||||
| paecilin B | ||||
| (5S, 10aR)-gonytolide C | ||||
| T-toxin | Bipolaris heterostrophus | [136] | ||
| cochliomycin A | antibacterial | Bipolaris lunatus (TA26-46) | [137] | |
| cochliomycin B | ||||
| cochliomycin C | ||||
| cochliomycin D | antifouling | |||
| cochliomycin E | ||||
| cochliomycin F | antifouling | |||
| zeaenol | ||||
| LL-Z1640-1 | cytotoxicity, antifouling | |||
| LL-Z1640-2 | fungicide | |||
| paecilomycin F | ||||
| (70E)-60-oxozeaenol | antifouling | |||
| (70E)-60-oxozeaenol antifouling | ||||
| aigialomycin B | ||||
| deoxy-aigialomycin C | antifouling | |||
| cochliobolic acid | Bipolaris lunatus | [138] | ||
| ethyl 2-[(3,5-dihydroxy)phenyl] acetate | Bipolaris sp. PSU-ES64 | [135] | ||
| (2-carboxy-3-hydroxy-5-methoxyphenyl) acetic acid | ||||
| 4-epiradicinol | antibacterial | |||
| radicinol | phytotoxicity | Bipolaris lunata | [139] | |
| epi-radicinol | phytotoxicity | |||
| radicinin | phytotoxicity | Bipolaris sp. | [140] | |
| lunatoic acid A | antifungal | Bipolaris lunata | [141] | |
| lunatoic acid B | ||||
| a-acetylorcinol | ||||
| Quinones | cynodontin | Curvularia lunata | [142] | |
| lunatin | Curvularia lunata | [143] | ||
| cytoskyrin A | ||||
| mitorubrinic acid A | Bipolaris lunatus | [144] | ||
| mitorubrinic acid B | ||||
| chrysophanol | Bipolaris sp. PSU-ES64 | [135] | ||
| emodin | ||||
| Terpenes | abscisic acid | Curvularia lunata | [143] | |
| zaragozic acid A | Curvularia lunata | [145] | ||
| 3a-hydroxy-5b-chol- 11-en-24-oic acid | Curvularia sp. | [146] | ||
| cochlioquinone A | leishmanicidal | Bipolaris sp. | [70] | |
| isocochlioquinone A | leishmanicidal | |||
| cochlioquinone B | Bipolaris miyabeanus | [124] | ||
| isocochlioquinone C | Bipolaris oryzae | [147] | ||
| anhydrocochlioquinone A | ||||
| ophiobolin A | cytotoxicity, antimalarial | Bipolaris heterostrophus race O | [148] | |
| 3-anhydroophiobolin A | Bipolaris oryzae | [147] | ||
| 6-epi-ophiobolin A | ||||
| 6-epi-3-anhydroophiobolin A | ||||
| ophiobolin B | ||||
| -epi-3-anhydroophiobolin B | ||||
| ophiobolin I | ||||
| 3-anhydro-6-hydroxy-ophiobolin A | antiproliferative | Bipolaris oryzae | [149] | |
| cis-sativenediol | Bipolaris setariae | [102] | ||
| isosativendiol | ||||
| IFO 6635 aversion factor | antifungal | Bipolaris setariae | [150] | |
| isosativenetriol | anti-inflammatory, anti-diabetic | Cochliobolus sp. | [71] | |
| anhydrocochlioquinone A | anti-tumor compound | Bipolaris oryzae | [147,151] | |
| cochlioquinone A1 | anti-angiogenic agent | Bipolaris zeicola (FIP 532) | [151,152], | |
| cochlioquinone A1 | anti-angiogenic agent | B. zeicola (AR5166) | [151,152] | |
| cochlioquinone A1 | anti-angiogenic agent | B. zeicola (AR 5168) | [151,152] | |
| cochlioquinones A, B | Cochliobolus miyabeanus | [124] | ||
| spiciferone A | antiviral property | Cochliobolus spicifer | [153] | |
| cochlioquinone A, isocochlioquinone A | leishmanicidal activity | Cochliobolus sp. | [70] |
| Species | Sources | Biological Activities | References |
|---|---|---|---|
| Curvularia lunata | Cymbopogon caesius | Antibacterial: Staphylococcus aureus. Antifungal: Candida albicans | [156] |
| Curvularia sp. | Catharanthus roseus | Antioxidant | [22] |
| Curvularia sp. | Phyllostachys edulis | Antibacterial: Bacillus subtilis, Listeria monocytogenes, Salmonella bacteria. Antifungal: Candida albicans. | [157] |
| Curvularia sp. | Moso bamboo (seeds) | Antibacterial: Listeria monocytogenes, Salmonella bacteria. Antifungal: Candida albicans. | [157] |
| Curvularia sp. (D12) | Garcinia spp. | Antibacterial: Mycobacterium tuberculosis | [158] |
| Curvularia lunata | Litchi chinensis | Phytotoxicity | [100,127] |
| Curvularia tuberculata | Marine algea | Antibacterial: Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa. Antioxidant | [159] |
| Curvularia pallescens | Laguncularia racemosa | Antibacterial: Staphylococcus aureus, Bacillus subtilis, Micrococcus luteus, Escherichia coli | [160] |
| Curvularia spp. | Ipomoea carnea | Leishmanicidal | [70] |
| Bipolaris geniculatus, B. spicifer | Cynodon dactylon | Antibacterial: Enterococcus faecalis | [161] |
| B. lunatus | Dactyloctenium aegyptium | Antibacterial: Salmonella enterica, Staphylococcus aureus. Antifungal: Candida albicans. Antioxidant | [161] |
| B. hawaiiensis | Antibacterial: Salmonella enterica, Staphylococcus aureus. Antifungal: Candida albicans. Antioxidant | [161] | |
| Bipolaris | Costus spiralis | Antibacterial: Pseudomonas aeruginosa, Salmonella enterica subsp. enterica serovar Typhi, Bacillus subtilis, and Enterococcus faecalis. Antifungal: Candida albicans, C. parapsilosis. Antioxidant | [162] |
| Bipolaris | Plumeria obtusifolia | Antibacterial: Pseudomonas aeruginosa. | [163] |
| Species | Accession Number | Host/Plant | References |
|---|---|---|---|
| Cochliobolus sp. | DQ836798 | Grapevine | [140] |
| Bipolaris ravenelii | Sporobolus spp. | [182] | |
| Bipolaris setariae | Eleusine indica | [183] | |
| Bipolaris sp. (B. zeicola) | GU046562 | Microstegium vimineum | [184] |
| Curvularia eragrostidis | Digitaria sanguinalis | [185] | |
| Curvularia lunata | Alisma plantago-aquatica | [186] | |
| Curvularia lunata | Echinochola spp. | [187] | |
| Curvularia tuberculata | Cyperus difformis | [188] | |
| Cyperus iridis | [188] | ||
| Fimbristylis miliacea | [188] | ||
| Cochliobolus australiensis | JX960591 | Pennisetum ciliare, or Cenchrus ciliaris | [27,100] |
| Name | Strain | Assembly | Level | Size (Mb) | GC% | Scaffolds | CDS | Genes | rRNA | tRNA |
|---|---|---|---|---|---|---|---|---|---|---|
| Bipolaris cookei | LSLP18.3 | GCA_002286855.1 | Scaffold | 36.1 | 49.7 | 320 | 0 | 0 | 0 | 0 |
| Bipolaris sorokiniana | BS112 | GCA_004329375.1 | Contig | 37.3 | 49.4 | 43 | 0 | 0 | 0 | 0 |
| Bipolaris sorokiniana | Shoemaker | GCA_013416765.1 | Scaffold | 34.3 | 48.1 | 96 | 10,755 | 10,755 | 0 | 0 |
| Bipolaris sorokiniana | ND90Pr | GCA_000338995.1 | Scaffold | 34.4 | 49.8 | 154 | 12,210 | 12,304 | 0 | 94 |
| Bipolaris oryzae | TG12bL2 | GCA_001675385.1 | Scaffold | 31.6 | 50.5 | 1640 | 0 | 0 | 0 | 0 |
| Bipolaris oryzae | ATCC 44560 | GCA_000523455.1 | Scaffold | 31.3 | 50.5 | 619 | 12,002 | 12,002 | 0 | 0 |
| Bipolaris victoriae | FI3 | GCA_000527765.1 | Scaffold | 32.8 | 50.1 | 676 | 12,882 | 12,882 | 0 | 0 |
| Bipolaris zeicola | GZL10 | GCA_016906865.1 | Contig | 36.1 | 50.6 | 23 | 0 | 0 | 0 | 0 |
| Bipolaris zeicola | Cocca1 | GCA_023079205.1 | Scaffold | 31.2 | 50.8 | 828 | 0 | 0 | 0 | 0 |
| Bipolaris maydis | ATCC 48331 | GCA_000354255.1 | Scaffold | 32.9 | 50.7 | 207 | 12,705 | 12,809 | 0 | 104 |
| Bipolaris zeicola | 26-R-13 | GCA_000523435.1 | Scaffold | 31.2 | 50.8 | 844 | 12,853 | 12,853 | 0 | 0 |
| Bipolaris maydis | BM1 | GCA_019454015.1 | Contig | 36.2 | 49.1 | 27 | 11,026 | 11,162 | 26 | 110 |
| Bipolaris maydis | KET7 | GCA_023087585.1 | Scaffold | 43.9 | 54.2 | 278 | 0 | 0 | 0 | 0 |
| Bipolaris maydis | C5 | GCA_000338975.1 | Scaffold | 36.4 | 49.8 | 68 | 13,316 | 13,456 | 0 | 140 |
| Bipolaris sorokiniana | WAI2411 | GCA_008452715.1 | Chromosome | 36.2 | 49.4 | 21 | 0 | 0 | 0 | 0 |
| Bipolaris sorokiniana | WAI2406 | GCA_008452705.1 | Chromosome | 36.8 | 49.4 | 21 | 0 | 0 | 0 | 0 |
| Bipolaris sorokiniana | BRIP10943a | GCA_008452735.1 | Chromosome | 36.9 | 49.5 | 22 | 0 | 0 | 0 | 0 |
| Bipolaris sorokiniana | BRIP27492a | GCA_008452725.1 | Chromosome | 35.2 | 49.4 | 19 | 0 | 0 | 0 | 0 |
| Curvularia sp. | IFB-Z10 | GCA_002161795.1 | Scaffold | 33.0 | 50.4 | 136 | 0 | 0 | 0 | 0 |
| Curvularia sp. | ZM96 | GCA_022457065.1 | Contig | 35.5 | 50.7 | 54 | 0 | 0 | 0 | 0 |
| Curvularia geniculata | P1 | GCA_016162275.1 | Contig | 32.9 | 50.7 | 753 | 0 | 0 | 0 | 0 |
| Curvularia geniculata | W3 | GCA_002982235.1 | Scaffold | 33.5 | 50.6 | 737 | 0 | 0 | 0 | 0 |
| Curvularia papendorfii | UM 226 | GCA_000817285.1 | Contig | 33.3 | 50.6 | 374 | 0 | 0 | 0 | 0 |
| Curvularia lunata | W3 | GCA_005212705.1 | Scaffold | 33.5 | 50.6 | 737 | 0 | 0 | 0 | 0 |
| Curvularia kusanoi | 30M1 | GCA_011058905.1 | Scaffold | 33.3 | 52.1 | 107 | 11,490 | 11,686 | 0 | 196 |
| Curvularia eragrostidis | C52 | GCA_020744315.1 | Contig | 36.8 | 52.1 | 3600 | 0 | 0 | 0 | 0 |
| Curvularia lunata | CX-3 | GCA_000743335.1 | Scaffold | 35.4 | 50.1 | 340 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.A.; Asaf, S.; Ahmad, W.; Jan, R.; Bilal, S.; Khan, I.; Khan, A.L.; Kim, K.-M.; Al-Harrasi, A. Diversity, Lifestyle, Genomics, and Their Functional Role of Cochliobolus, Bipolaris, and Curvularia Species in Environmental Remediation and Plant Growth Promotion under Biotic and Abiotic Stressors. J. Fungi 2023, 9, 254. https://doi.org/10.3390/jof9020254
Khan NA, Asaf S, Ahmad W, Jan R, Bilal S, Khan I, Khan AL, Kim K-M, Al-Harrasi A. Diversity, Lifestyle, Genomics, and Their Functional Role of Cochliobolus, Bipolaris, and Curvularia Species in Environmental Remediation and Plant Growth Promotion under Biotic and Abiotic Stressors. Journal of Fungi. 2023; 9(2):254. https://doi.org/10.3390/jof9020254
Chicago/Turabian StyleKhan, Nasir Ali, Sajjad Asaf, Waqar Ahmad, Rahmatullah Jan, Saqib Bilal, Ibrahim Khan, Abdul Latif Khan, Kyung-Min Kim, and Ahmed Al-Harrasi. 2023. "Diversity, Lifestyle, Genomics, and Their Functional Role of Cochliobolus, Bipolaris, and Curvularia Species in Environmental Remediation and Plant Growth Promotion under Biotic and Abiotic Stressors" Journal of Fungi 9, no. 2: 254. https://doi.org/10.3390/jof9020254