Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies
Abstract
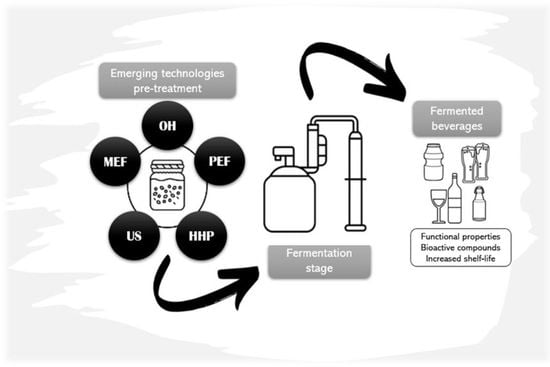
:1. Introduction
2. Fermented Beverages Development
3. Emerging Technologies as Fermentation-Assisted Processes for Fermented Beverages Development
3.1. Ohmic Heating, Moderate Electric Fields and Pulsed Electric Fields
3.2. Ultrasound
3.3. High Hydostratic Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul Ross, R.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2019, 36, 135–167. [Google Scholar] [CrossRef]
- Navarrete-Bolaños, J.L. Improving traditional fermented beverages: How to evolve from spontaneous to directed fermentation. Eng. Life Sci. 2012, 12, 410–418. [Google Scholar] [CrossRef]
- Taveira, I.C.; Nogueira, K.M.V.; Oliveira, D.L.G.D.; Silva, R.D.N. Fermentation: Humanity’s oldest biotechnological tool. Front. Young Minds 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Puerta, G.I.; Fundamentos del proceso de fermentación en el beneficio del café. Centro Nacional de Investigaciones de Café (Cenicafé). 2013. Available online: https://www.cenicafe.org/es/publications/avt0402.pdf (accessed on 30 November 2022).
- Ray, B.; Daeschel, M. Food Biopreservatives of Microbial Origin; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Robinson, T.; Singh, D.; Nigam, P. Fermentación en estado sólido: Una tecnología microbiana promisoria para la producción de metabolitos secundarios. Vitae Rev. Fac. Química Farm. 2002, 9, 27–36. [Google Scholar]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Caplice, E. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Njobeh, P.B.; Adeboye, A.S.; Adebiyi, J.A.; Sobowale, S.S.; Ogundele, O.M.; Kayitesi, E. Advances in Fermentation Technology for Novel Food Products. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer International Publishing: Cham, Switzerland, 2018; pp. 71–87. [Google Scholar]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.-L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. NRR 2017, 30, 1–24. [Google Scholar] [CrossRef]
- García Palancar, M. Bebidas fermentadas y probióticos: ¿cervezas probióticas? Bachelor’s Thesis, Universidad Complutense de Madrid, Madrid, España, 2017. [Google Scholar]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar]
- Hager, A.-S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer—A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Maize and Sorghum as Raw Materials for Brewing, a Review. Appl. Sci. 2021, 11, 3139. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast Interactions in Inoculated Wine Fermentation. Front. Microbiol. 2016, 7, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles Calderón, R.; Feliciano Muñoz, O.; Chirre Flores, J.H. Estudio del consumo de azúcares reductores durante la fermentación alcohólica del mosto de uva Italia para la obtención de vino blanco. Ind. Data 2016, 19, 104. [Google Scholar] [CrossRef] [Green Version]
- Čakar, U.; Petrović, A.; Pejin, B.; Čakar, M.; Živković, M.; Vajs, V.; Đorđević, B. Fruit as a substrate for a wine: A case study of selected berry and drupe fruit wines. Sci. Hortic. 2019, 244, 42–49. [Google Scholar] [CrossRef]
- Maldonado, R.R.; de Oliveira, D.S.; Alves, V.D.; Oliveira, E.A.; Kamimura, E.S. Application of tamarind pulp for wine production. J. Biotechnol. Biodiver. 2021, 9, 163–169. [Google Scholar] [CrossRef]
- Rosend, J.; Kaleda, A.; Kuldjärv, R.; Arju, G.; Nisamedtinov, I. The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product. Foods 2020, 9, 1401. [Google Scholar] [CrossRef]
- Parra Parra, F.T. Evaluación de las características organolépticas de la sidra procedente de la manzana Emilia (reineta amarilla de blenheím) en su crianza con chips de roble. Bachelor’s Thesis, Universidad del Azuay, Azuay, Ecuador, 2013. [Google Scholar]
- Estela-Escalante, W.D.; Rychtera, M.; Melzoch, K.; Torres-Ibáñez, F.; Calixto-Cotos, R.; Bravo-Araníbar, N.; Memenza-Zegarra, M.E.; Chávez-Guzmán, Y.M. Efecto de la aireación en la producción de compuestos volátiles por cultivo mixto de Brettanomyces intermedius y Saccharomyces cerevisiae durante la fermentación de cidra. TIP 2014, 17, 5–14. [Google Scholar] [CrossRef]
- Hou, C.Y.; Huang, P.H.; Lai, Y.T.; Lin, S.P.; Liou, B.K.; Lin, H.W.; Hsieh, C.-W.; Cheng, K.C. Screening and identification of yeasts from fruits and their coculture for cider production. Fermentation 2022, 8, 1. [Google Scholar] [CrossRef]
- Miranda-Mejía, G.A. Impact of Pulsed Electric Fields on Fermentation Process during Yogurt Production. Master’s Thesis, Tecnológico de Monterrey, Querétaro, Mexico, 2022. [Google Scholar]
- Taş, T.K.; Ekinci, F.Y.; Guzel-Seydim, Z.B. Identification of microbial flora in kefir grains produced in Turkey using PCR. Int. J. Dairy Technol. 2011, 65, 126–131. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-based alternatives to yogurt: State-of-the-art and perspectives of new biotechnological challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Comp. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef] [PubMed]
- Aydar, A.Y.; Mataracı, C.E.; Sağlam, T.B. Development and modeling of a novel plant-based yoghurt produced by Jerusalem artichoke and almond milk using l-optimal mixture design. J. Food Meas. Charact. 2021, 15, 3079–3087. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. CRFSFS 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Talebi, M.; Frink, L.A.; Patil, R.A.; Armstrong, D.W. Examination of the varied and changing ethanol content of commercial kombucha products. Food Anal. Methods 2017, 10, 4062–4067. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.X.; Mutukumira, A.N. Kombucha: Production and Microbiological Research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Gavahian, M.; Mathad, G.N.; Oliveira, C.A.; Khaneghah, A.M. Combinations of emerging technologies with fermentation: Interaction effects for detoxification of mycotoxins? Food Res. Int. 2021, 141, 110104. [Google Scholar] [CrossRef]
- Yıldız, G.; Yildiz, G.; Khan, M.R.; Aadil, R.M. High-intensity ultrasound treatment to produce and preserve the quality of fresh-cut kiwifruit. J. Food Process. Preserv. 2022, 46, e16542. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.-A.; Aadil, R.M.; Trif, M. Applications of innovative non-thermal pulsed electric field technology in developing safer and healthier fruit juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef]
- Roobab, U.; Khan, A.W.; Irfan, M.; Madni, G.M.; Zeng, X.A.; Nawaz, A.; Walayat, N.; Manzoor, M.F.; Aadil, R.M. Recent developments in ohmic technology for clean label fruit and vegetable processing: An overview. J. Food Process. Eng. 2022, 45, e14045. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Arshad, R.N.; Roobab, U.; Yaseen, B.; Ranjha, M.M.A.N.; Aadil, R.M.; Ibrahim, S.A. Potential Impact of Ultrasound, Pulsed Electric Field, High-Pressure Processing, Microfludization Against Thermal Treatments Preservation Regarding Sugarcane Juice (Saccharum officinarum). Ultrason. Sonochem. 2022, 90, 106194. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Liu, J.; Li, C.; Li, J.; Zhu, D. Plant-Based Fermented Beverages and Key Emerging Processing Technologies. Food. Rev. Int. 2022, 1–20. [Google Scholar] [CrossRef]
- García Martín, J.F.; Sun, D.-W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, H.; Kim, M.; Han, H.; Kim, W.J. Effect of ultrasonication on fermentation kinetics of beer using six-row barley cultivated in Korea. J. Inst. Brew. 2015, 121, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Hulbert, G.J.; Mount, J.R. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov. Food Sci. Emerg. Technol. 2000, 1, 211–218. [Google Scholar] [CrossRef]
- Barukčić, I.; Lisak Jakopović, K.; Herceg, Z.; Karlović, S.; Božanić, R. Influence of high intensity ultrasound on microbial reduction, physico-chemical characteristics and fermentation of sweet whey. IFSET 2015, 27, 94–101. [Google Scholar] [CrossRef]
- Liu, L.; Loira, I.; Morata, A.; Suárez-Lepe, J.A.; González, M.C.; Rauhut, D. Shortening the ageing on lees process in wines by using ultrasound and microwave treatments both combined with stirring and abrasion techniques. Eur. Food Res. Technol. 2015, 242, 559–569. [Google Scholar] [CrossRef]
- Vazquez-Cabral, D.; Valdez-Fragoso, A.; Rocha-Guzman, N.E.; Moreno-Jimenez, M.R.; Gonzalez-Laredo, R.F.; Morales-Martinez, P.S.; Rojas-Contreras, J.A.; Mujica-Paz, H.; Gallegos-Infante, J.A. Effect of pulsed electric field (PEF)-treated kombucha analogues from Quercus obtusata infusions on bioactives and microorganisms. IFSET 2016, 34, 171–179. [Google Scholar] [CrossRef]
- El Darra, N.; Turk, M.F.; Ducasse, M.-A.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Changes in polyphenol profiles and color composition of freshly fermented model wine due to pulsed electric field, enzymes and thermovinification pretreatments. Food Chem. 2016, 194, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Daryaei, H.; Coventry, J.; Versteeg, C.; Sherkat, F. Combined pH and high hydrostatic pressure effects on Lactococcus starter cultures and Candida spoilage yeasts in a fermented milk test system during cold storage. Food Microbiol. 2010, 27, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, M.A.; Loira, I.; Escott, C.; Del Fresno, J.M.; Morata, A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape Processing by High Hydrostatic Pressure: Effect on Use of Non-Saccharomyces in Must Fermentation. Food Bioprocess. Technol. 2016, 9, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Xiao, L.; Tahir, H.E. Aroma profile and sensory characteristics of a sulfur dioxide-free mulberry (Morus nigra) wine subjected to non-thermal accelerating aging techniques. Food Chem. 2017, 232, 89–97. [Google Scholar] [CrossRef]
- Mota, M.J.; Lopes, R.P.; Koubaa, M.; Roohinejad, S.; Barba, F.J.; Delgadillo, I.; Saraiva, J.A. Fermentation at non-conventional conditions in food-and bio-sciences by the application of advanced processing technologies. Crit. Rev. Biotech. 2018, 38, 122–140. [Google Scholar] [CrossRef]
- Castro, I.; Teixeira, J.; Vicente, A. The effect of the electric field on lag-phase, ethanol and β-galactosidase production of a recombinant S. cerevisiae growing on lactose. 2nd Mercosur Congr. Chem. Eng. 2005. [Google Scholar]
- Gavahian, M.; Farahnaky, A. Ohmic-assisted hydrodistillation technology: A review. Trends Food Sci. Technol. 2018, 72, 153–161. [Google Scholar] [CrossRef]
- Gally, T.; Rouaud, O.; Jury, V.; Havet, M.; Og´e, A.; Le-Bail, A. Proofing of bread dough assisted by ohmic heating. Innov. Food Sci. Emerg. Technol. 2017, 39, 55–62. [Google Scholar] [CrossRef]
- Knirsch, M.C.; Alves dos Santos, C.; Martins de Oliveira Soares Vicente, A.A.; Vessoni Penna, T.C. Ohmic heating—A review. Trends Food Sci. Technol. 2010, 21, 436–441. [Google Scholar] [CrossRef]
- Alcántara-Zavala, A.E.; de Dios Figueroa-Cárdenas, J.; Morales-Sánchez, E.; Aldrete-Tapia, J.A.; Arvizu-Medrano, S.M.; Martínez-Flores, H.E. Application of ohmic heating to extend shelf life and retain the physicochemical, microbiological, and sensory properties of pulque. Food Bioprod. Process. 2019, 118, 139–148. [Google Scholar] [CrossRef]
- Silva, A.B.; Scudini, H.; Ramos, G.L.P.; Pires, R.P.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Margalho, L.P.; Pimentel, T.C.; Siva, M.C.; et al. Ohmic heating processing of milk for probiotic fermented milk production: Survival kinetics of Listeria monocytogenes as contaminant post-fermentation, bioactive compounds retention and sensory acceptance. Int. J. Food Microbiol. 2021, 348, 109204. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; la Peña, M.M.-d.; Welti-Chanes, J.; Guerrero-Beltrán, J.Á. Pulsed electric field processing of a pomegranate (Punica granatum L.) Fermented beverage. IFSET 2022, 79, 103045. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Microbial Decontamination of Red Wine by Pulsed Electric Fields (PEF) after Alcoholic and Malolactic Fermentation: Effect on Saccharomyces cerevisiae, Oenococcus oeni, and Oenological Parameters during Storage. Foods 2023, 12, 278. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Vorobiev, E.; Maroun, R.G.; Louka, N. Control of the sugar/ethanol conversion rate during moderate pulsed electric field-assisted fermentation of a Hanseniaspora sp. Strain to produce low-alcohol cider. IFSET 2020, 59, 102258. [Google Scholar] [CrossRef]
- El Darra, N.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Foods Res. Technol. 2012, 236, 47–56. [Google Scholar] [CrossRef]
- Saldaña, G.; Cebrián, G.; Abenoza, M.; Sánchez-Gimeno, C.; Álvarez, I.; Raso, J. Assessing the efficacy of PEF treatments for improving polyphenol extraction during red wine vinifications. IFSET 2017, 39, 179–187. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Versari, A. Recent Advances and Applications of Pulsed Electric Fields (PEF) to Improve Polyphenol Extraction and Color Release during Red Winemaking. Beverages 2018, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Chanos, P.; Warncke, M.C.; Ehrmann, M.A.; Hertel, C. Application of mild pulsed electric fields on starter culture accelerates yogurt fermentation. Eur. Foods Res. Technol. 2020, 246, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Rodríguez, M. Incorporation of Microencapsulated Iron in Dried Chili Mangoes (Mangifera indica L. Var. Ataulfo) with ultrasound pre-treatment. Master’s Thesis, Tecnológico de Monterrey, Querétaro, Mexico, 2022. [Google Scholar]
- Zhang, Y.; Abatzoglou, N. Review: Fundamentals, applications and potentials of ultrasound-assisted drying. Chem. Eng. Res. Des. 2020, 154, 21–46. [Google Scholar] [CrossRef]
- Galván-D’Alessandro, L.; Carciochi, R. Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review. Fermentation 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Umego, E.C.; He, R.; Huang, G.; Dai, C.; Ma, H. Ultrasound-assisted fermentation: Mechanisms, technologies, and challenges. J. Food Process. Preserv. 2021, 45, e15559. [Google Scholar] [CrossRef]
- Ojha, K.S.; Kerry, J.P.; Alvarez, C.; Walsh, D.; Tiwari, B.K. Effect of high intensity ultrasound on the fermentation profile of Lactobacillus sakei in a meat model system. Ultrason. Sonochem. 2016, 31, 539–545. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Lee, Y.K.; Zhou, W. Stimulating fermentative activities of bifidobacteria in milk by highintensity ultrasound. Int. Dairy J. 2009, 19, 410–416. [Google Scholar] [CrossRef]
- Liu, W.-S.; Yang, C.-Y.; Fang, T.J. Strategic ultrasound-induced stress response of lactic acid bacteria on enhancement of β-glucosidase activity for bioconversion of isoflavones in soymilk. J. Microbiol. Methods 2018, 148, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent insights in the impact of emerging technologies on lactic acid bacteria: A review. Food Res. Int. 2020, 137, 109544. [Google Scholar] [CrossRef]
- Carrillo-Lopez, L.M.; Garcia-Galicia, I.A.; Tirado-Gallegos, J.M.; Sanchez-Vega, R.; Huerta-Jimenez, M.; Ashokkumar, M.; Alarcon-Rojo, A.D. Recent advances in the application of ultrasound in dairy products: Effect on functional, physical, chemical, microbiological and sensory properties. Ultrason. Sonochem. 2021, 73, 105467. [Google Scholar] [CrossRef]
- Abesinghe, A.M.N.L.; Islam, N.; Vidanarachchi, J.K.; Prakash, S.; Silva, K.F.S.T.; Karim, M.A. Effects of ultrasound on the fermentation profile of fermented milk products incorporated with lactic acid bacteria. Int. Dairy J. 2019, 90, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nöbel, S.; Ross, N.-L.; Protte, K.; Körzendörfer, A.; Hitzmann, B.; Hinrichs, J. Microgel particle formation in yogurt as influenced by sonication during fermentation. J. Food Eng. 2016, 180, 29–38. [Google Scholar] [CrossRef]
- Gavahian, M.; Manyatsi, T.S.; Morata, A.; Tiwari, B.K. Ultrasound-assisted production of alcoholic beverages: From fermentation and sterilization to extraction and aging. CRFSFS 2022, 21, 5243–5271. [Google Scholar] [CrossRef]
- Tao, Y.; García, J.F.; Sun, D.-W. Advances in Wine Aging Technologies for Enhancing Wine Quality and Accelerating Wine Aging Process. Crit. Rev. Food Sci. Nutr. 2013, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gómez-Plaza, E. Using high-power ultrasounds in red winemaking: Effect of operating conditions on wine physico-chemical and chromatic characteristics. LWT 2021, 138, 110645. [Google Scholar] [CrossRef]
- Herrera-Ponce, A.L.; Salmeron-Ochoa, I.; Rodriguez-Figueroa, J.C.; Santellano-Estrada, E.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D. High-intensity ultrasound as pre-treatment in the development of fermented whey and oat beverages: Effect on the fermentation, antioxidant activity and consumer acceptance. JFST 2021, 59, 796–804. [Google Scholar] [CrossRef]
- Simon-Sarkadi, L.; Pásztor-Huszár, K.; Dalmadi, I.; Kiskó, G. Effect of high hydrostatic pressure processing on biogenic amine content of sausage during storage. Int. Food Res. J. 2012, 47, 380–384. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Balasubramaniam, V.M. Fundamentals and Applications of High-Pressure Processing Technology. In High Pressure Processing of Food; Balasubramaniam, V.M., Barbosa-Canovas, G.V., Lelieveld, H., Eds.; Springer: New York, NY, USA, 2016; pp. 3–17. [Google Scholar]
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Novel technologies to improve food safety and quality. Curr. Opin. Food Sci. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Chen, Y.; Feng, X.; Wu, C.; Long, F. Purified Saponins in Momordica charantia Treated with High Hydrostatic Pressure and Ionic Liquid-Based Aqueous Biphasic Systems. Foods 2022, 11, 1930. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Zhao, M.; Tong, P.; Lv, L.; Gao, Z.; Liu, J.; Long, F. High Hydrostatic Pressure Treatments Improved Properties of Fermentation of Apple Juice Accompanied by Higher Reserved Lactobacillus plantarum. Foods 2023, 12, 441. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Welti-Chanes, J.; Rodríguez-Martínez, V.; Guerrero-Beltrán, J.Á. Influence of high hydrostatic pressure processing on physicochemical characteristics of a fermented pomegranate (Punica granatum L.) Beverage. IFSET 2020, 59, 102249. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Tang, D.; Zhang, Y. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. IFSET 2014, 23, 61–67. [Google Scholar] [CrossRef]
- Pega, J.; Denoya, G.I.; Castells, M.L.; Sarquis, S.; Aranibar, G.F.; Vaudagna, S.R.; Nanni, M. Effect of High-Pressure Processing on Quality and Microbiological Properties of a Fermented Beverage Manufactured from Sweet Whey Throughout Refrigerated Storage. Food Bioprocess Technol. 2018, 11, 1101–1110. [Google Scholar] [CrossRef]
- De Ancos, B.; Pilar Cano, M.; Gómez, R. Characteristics of stirred low-fat yoghurt as affected by high pressure. Int. Dairy J. 2000, 10, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Swelam, S. Impact of High Hydrostatic Pressure on Composition and Quality of Yoghurt. JFDS 2018, 9, 31–35. [Google Scholar] [CrossRef]

| Processing Technology | Fermented Product | Treatment Parameters | Microorganisms | Main Results | Reference |
|---|---|---|---|---|---|
| US | Beer | 400 kHz & 160 W | S. cerevisiae | Improved ethanol production by 13.18% | [43] |
| Full-fat yogurt | 90, 225 & 450 W-6 min | S. thermophilus, Lb. bulgaricus, Lb. acidophilus | Higher water holding capacity, viscosity, less syneresis. Fermentation time reduction (30 min) | [44] | |
| Sweet whey | 84 W-150 s | Lb. acidophilus La-5 | Fermentation time reduction (30 min) with 1 log cycle higher colony count than the control | [45] | |
| Wine | 50 kHz & 200 W | S. cerevisiae | Aging time reduction | [46] | |
| PEF | Natural drinkable yogurt | 3 kV/cm, 150 Hz, 400-μs | S. thermophilus. Lb. bulgaricuss | Fermentation time reduction (42 min) | [25] |
| Kombucha analogs | 37.3-53.4 kV/cm, 445.3–1979.2 μs | Kombucha consortium | Inactivation of acetic acid bacteria | [47] | |
| Wine | 5 kV/cm, 1 ms, 100 pulses of 10-μs, 0.5 Hz | S. cerevisiae | Increase 41% total phenolic compounds, 56% color intensity, and 48% flavonols | [48] | |
| HPP | Fermented milk | 300 & 600 MPa | Lb. lactis | Reduced viable counts of Candida spoilage yeasts | [49] |
| Must | 400 MPa | S. cerevisiae, Schizosaccharomyces pombe, Torulaspora delbrueckii, Metschnikowia pulcherrima. Lachancea thermotolerans. | Reduced/eliminated microbial counts | [50] | |
| Wine | 300 MPa, 20 min | S. cerevisiae | Increase concentration of esters, aldehydes, ketones, terpenes, lactones, and furans | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-de la Peña, M.; Miranda-Mejía, G.A.; Martín-Belloso, O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages 2023, 9, 51. https://doi.org/10.3390/beverages9020051
Morales-de la Peña M, Miranda-Mejía GA, Martín-Belloso O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages. 2023; 9(2):51. https://doi.org/10.3390/beverages9020051
Chicago/Turabian StyleMorales-de la Peña, Mariana, Graciela A. Miranda-Mejía, and Olga Martín-Belloso. 2023. "Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies" Beverages 9, no. 2: 51. https://doi.org/10.3390/beverages9020051