Effects of Amazake Produced with Different Aspergillus on Gut Barrier and Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Manufacture of Koji Amazake
2.3. Measurement of Sugars Composition in Amazake
2.4. Measurement of Organic Acids Composition in Amazake
2.5. Measurement of Ferulic Acid in Amazake
2.6. Measurement of Dietary Fiber in Amazake
2.7. Animals Experiment Design
2.8. Measurement of Biochemical Indexes in the Animal Experiment
2.9. Gut Microbiota Analysis by 16S rRNA Gene Sequencing
2.10. Statistical Analysis
3. Results
3.1. Analysis of Amazake Ingredients
3.2. Effects of Amazake on Expressions of Gut Antioxidant Proteins
3.3. Effects of Amazake on the Expression of Gut Barrier Protein and Inflammation Factors
3.4. Effects of Amazake on Gut Microbiota Structure
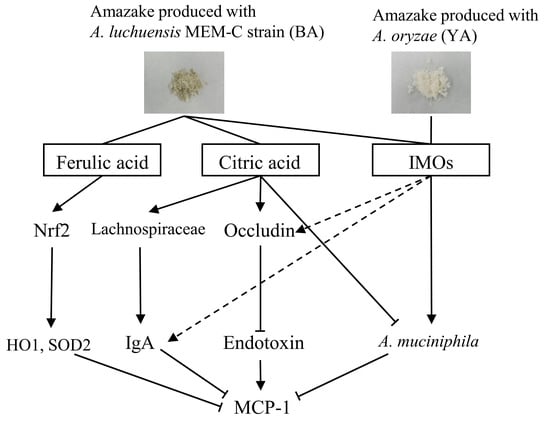
4. Discussion
4.1. Difference between the Two Types of Amazake Ingredients
4.2. Effects of Amazake on Gut Barrier Function and Gut Microbiota
4.3. Relationship between Gut Barrier Function and Gut Microbiota
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Components (%) | ND | WD | ||
|---|---|---|---|---|
| ー | ー | BA | YA | |
| Lard | 3 | 30 | 29.7 | 29.7 |
| Soybean oil | 3 | 3 | 3 | 3 |
| Corn starch | 43 | 15 | 5.9 | 5.9 |
| Casein | 21 | 21 | 20.4 | 20.4 |
| Sucrose | 20 | 20 | 20 | 20 |
| Cellulose | 5 | 5 | 5 | 5 |
| Mineral Mix | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin Mix | 1 | 1 | 1 | 1 |
| Cholesterol | 0 | 1 | 1 | 1 |
| Choline Bitartrate | 0.2 | 0.2 | 0.2 | 0.2 |
| Methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Black koji Amazake (BA) | 10 | |||
| Yellow koji Amazake (YA) | 10 | |||
| Total calories (kcal/100 g) | 371 | 520 | 523 | 523 |

References
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Parkes, G. IBD Statistics 2022: Crohn’s and Ulcerative Colitis. Available online: https://ampersandhealth.co.uk/myibdcare/resources/ibd-statistics-2022-crohns-and-ulcerative-colitis/#:~:text=Whatpercentageofpeopleget,WorldIBDDay2022here (accessed on 3 June 2023).
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- He, W.-Q.; Wang, J.; Sheng, J.-Y.; Zha, J.-M.; Graham, W.V.; Turner, J.R. Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis. Int. J. Mol. Sci. 2020, 21, 993. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, N.G.; Baranwal, S.; Khan, S.; Feygin, A.; Gupta, P.; Ivanov, A.I. Novel mechanism of cytokine-induced disruption of epithelial barriers. Tissue Barriers 2013, 1, e25231. [Google Scholar] [CrossRef]
- Tena-Garitaonaindia, M.; Arredondo-Amador, M.; Mascaraque, C.; Asensio, M.; Marin, J.J.G.; Martínez-Augustin, O.; Sánchez de Medina, F. Modulation of intestinal barrier function by glucocorticoids: Lessons from preclinical models. Pharmacol. Res. 2022, 177, 106056. [Google Scholar] [CrossRef]
- do Nascimento, R.D.P.; da Fonseca Machado, A.P.; Galvez, J.; Cazarin, C.B.B.; Maróstica Junior, M.R. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, H.Y.; Kim, T.K.; Kim, M.S.; Park, Y.M.; Kim, J.; Park, K.; Kweon, M.N.; Kim, S.H.; Bae, J.W.; et al. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS ONE 2017, 12, e0187515. [Google Scholar] [CrossRef]
- Oguro, Y.; Nishiwaki, T.; Shinada, R.; Kobayashi, K.; Kurahashi, A. Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. J. Biosci. Bioeng. 2017, 124, 178–183. [Google Scholar] [CrossRef]
- Michio Sata, Y.N. Effect of a Late Evening Snack of Amazake in Patients with Liver Cirrhosis: A Pilot Study. J. Nutr. Food Sci. 2013, 3, 1000223. [Google Scholar] [CrossRef]
- Maruki-Uchida, H.; Sai, M.; Yano, S.; Morita, M.; Maeda, K. Amazake made from sake cake and rice koji suppresses sebum content in differentiated hamster sebocytes and improves skin properties in humans. Biosci. Biotechnol. Biochem. 2020, 84, 1689–1695. [Google Scholar] [CrossRef]
- Oura, S.; Suzuki, S.; Hata, Y.; Kawato, A.; Abe, Y. Evaluation of physiological functionalities of amazake in mice. J. Brew. Soc. JAPAN 2007, 102, 781–788. [Google Scholar] [CrossRef]
- Saigusa, N.; Ohba, R. Effects of koji production and saccharification time on the antioxidant activity of amazake. Food Sci. Technol. Res. 2007, 13, 162–165. [Google Scholar] [CrossRef]
- Kawakami, S.; Ito, R.; Maruki-Uchida, H.; Kamei, A.; Yasuoka, A.; Toyoda, T.; Ishijima, T.; Nishimura, E.; Morita, M.; Sai, M.; et al. Intake of a mixture of sake cake and rice malt increases mucin levels and changes in intestinal microbiota in mice. Nutrients 2020, 12, 449. [Google Scholar] [CrossRef]
- International, A.; Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Rockville, MD, USA, 1995. [Google Scholar]
- Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.X. Bilberry anthocyanins ameliorate NAFLD by improving dyslipidemia and gut microbiome dysbiosis. Nutrients 2020, 12, 3252. [Google Scholar] [CrossRef]
- Kurahashi, A. Ingredients, Functionality, and Safety of the Japanese Traditional Sweet Drink Amazake. J. Fungi 2021, 7, 469. [Google Scholar] [CrossRef]
- Kadooka, C.; Nakamura, E.; Mori, K.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. LaeA Controls Citric Acid Production through Regulation of the Citrate Exporter-Encoding cexA Gene in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2020, 86, e01950-19. [Google Scholar] [CrossRef]
- Koseki, T.; Ito, Y.; Furuse, S.; Ito, K.; Iwano, K. Conversion of ferulic acid into 4-vinylguaiacol, vanillin and vanillic acid in model solutions of shochu. J. Ferment. Bioeng. 1996, 82, 46–50. [Google Scholar] [CrossRef]
- von Martels, J.Z.H.; Bourgonje, A.R.; Klaassen, M.A.Y.; Alkhalifah, H.A.A.; Sadaghian Sadabad, M.; Vich Vila, A.; Gacesa, R.; Gabriëls, R.Y.; Steinert, R.E.; Jansen, B.H.; et al. Riboflavin Supplementation in Patients with Crohn’s Disease [the RISE-UP study]. J. Crohn’s Colitis 2020, 14, 595–607. [Google Scholar] [CrossRef]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging Increases the Severity of Colitis and the Related Changes to the Gut Barrier and Gut Microbiota in Humans and Mice. J. Gerontol. Ser. A 2020, 75, 1284–1292. [Google Scholar] [CrossRef]
- Tsuruta, T.; Muhomah, T.A.; Sonoyama, K.; Nguyen, Q.D.; Takase, Y.; Nishijima, A.; Himoto, S.; Katsumata, E.; Nishino, N. Aicda deficiency exacerbates high-fat diet-induced hyperinsulinemia but not gut dysbiosis in mice. Nutr. Res. 2021, 93, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A Protective Role of the NRF2-Keap1 Pathway in Maintaining Intestinal Barrier Function. Oxid. Med. Cell Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef] [PubMed]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Ragab Farag, M.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, R.; Afzali, N. Effect of Citric Acid and Microbial Phytase on Small Intestinal Morphology in Broiler Chicken. Ital. J. Anim. Sci. 2013, 12, e7. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Zheng, J.; Dai, J.; Ou, W.; Xu, W.; Ai, Q.; Zhang, W.; Niu, J.; Mai, K.; et al. Citric acid mitigates soybean meal induced inflammatory response and tight junction disruption by altering TLR signal transduction in the intestine of turbot, Scophthalmus maximus L. Fish Shellfish Immunol. 2019, 92, 181–187. [Google Scholar] [CrossRef]
- Xue, J.J.; Huang, X.F.; Liu, Z.L.; Chen, Y.; Zhang, Y.K.; Luo, Y.; Wang, B.W.; Wang, Q.G.; Wang, C. Effects of citric acid supplementation on growth performance, intestinal morphology and microbiota, and blood parameters of geese from 1 to 28 days of age. Poult. Sci. 2023, 102, 102343. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Lv, L.; Wu, S.; Guo, Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE−/− mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2020, 27, 7910–7921. [Google Scholar] [CrossRef]
- Pan, H.; Feng, W.; Chen, M.; Luan, H.; Hu, Y.; Zheng, X.; Wang, S.; Mao, Y. Alginate Oligosaccharide Ameliorates D-Galactose-Induced Kidney Aging in Mice through Activation of the Nrf2 Signaling Pathway. Biomed Res. Int. 2021, 2021, 6623328. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Zhang, A.; Zhao, N.; Zhang, J.; Zhao, D.; Yu, Z.; Xu, N.; Yin, Y.; Luan, X.; et al. Oligosaccharide attenuates aging-related liver dysfunction by activating Nrf2 antioxidant signaling. Food Sci. Nutr. 2020, 8, 3872–3881. [Google Scholar] [CrossRef]
- Tao, W.; Wang, G.; Pei, X.; Sun, W.; Wang, M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants 2022, 11, 1384. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, J.; Boparai, R.K.; Zhu, J.; Mantri, S.; Khare, P.; Khardori, R.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 2017, 123, 103–113. [Google Scholar] [CrossRef]
- Van Herreweghen, F.; Van den Abbeele, P.; De Mulder, T.; De Weirdt, R.; Geirnaert, A.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Belzer, C.; et al. In vitro colonisation of the distal colon by Akkermansia muciniphila is largely mucin and pH dependent. Benef. Microbes 2017, 8, 81–96. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Zhao, R.; Shen, G.X. Impact of Anthocyanin Component and Metabolite of Saskatoon Berry on Gut Microbiome and Relationship with Fecal Short Chain Fatty Acids in Diet-Induced Insulin Resistant Mice. J. Nutr. Biochem. 2022, 111, 109201. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Q.; Liu, Y.; Shi, Z.; Hu, L.; Zeng, Z.; Tu, Y.; Xiao, Z.; Xu, Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J. Immunol. Res. 2021, 2021, 8816041. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2023, 13, 1055914. [Google Scholar] [CrossRef] [PubMed]
- Janzon, A.; Goodrich, J.K.; Koren, O.; Waters, J.L.; Ley, R.E. Interactions between the Gut Microbiome and Mucosal Immunoglobulins A, M, and G in the Developing Infant Gut. mSystems 2019, 4, e00612-19. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Drees, C.; Watson, A.R.; Plunkett, C.H.; Nagler, C.R.; Schneewind, O.; Eren, A.M.; Bendelac, A. B cell superantigens in the human intestinal microbiota. Sci. Transl. Med. 2019, 11, eaau9356. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Pérez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Duran, D.; D’Angelo, M.; Solà-Oriol, D. Targeted-Release Organic Acids and Essential Oils Improve Performance and Digestive Function in Broilers under a Necrotic Enteritis Challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef]




| Components | BA | YA |
|---|---|---|
| Ferulic acid (mg/100 g) | 0.55 | 0.008 |
| Dietary Fiber (g/100g) | 2.3 | 1.3 |
| Isomaltose (g/100 g) | 9.2 | 5.2 |
| Isomaltotriose (g/100 g) | 0.66 | 0.24 |
| Total organic acids (mg/100 g) | 1760 | 288 |
| Citric acid (mg/100 g) | 1553 | 76 |
| Pyroglutamic acid (mg/100 g) | 111 | 23 |
| Malic acid (mg/100 g) | 8.1 | 1.3 |
| Lactic acid (mg/100 g) | 2.7 | 31.9 |
| Acetic acid (mg/100 g) | 2.5 | 15.2 |
| pH | 3.80 | 6.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, H.; Setoguchi, S.; Kawano, K.; Miyagawa, H.; Sakao, K.; Hou, D.-X. Effects of Amazake Produced with Different Aspergillus on Gut Barrier and Microbiota. Foods 2023, 12, 2568. https://doi.org/10.3390/foods12132568
Nakano H, Setoguchi S, Kawano K, Miyagawa H, Sakao K, Hou D-X. Effects of Amazake Produced with Different Aspergillus on Gut Barrier and Microbiota. Foods. 2023; 12(13):2568. https://doi.org/10.3390/foods12132568
Chicago/Turabian StyleNakano, Hironobu, Sho Setoguchi, Kuniaki Kawano, Hiroshi Miyagawa, Kozue Sakao, and De-Xing Hou. 2023. "Effects of Amazake Produced with Different Aspergillus on Gut Barrier and Microbiota" Foods 12, no. 13: 2568. https://doi.org/10.3390/foods12132568