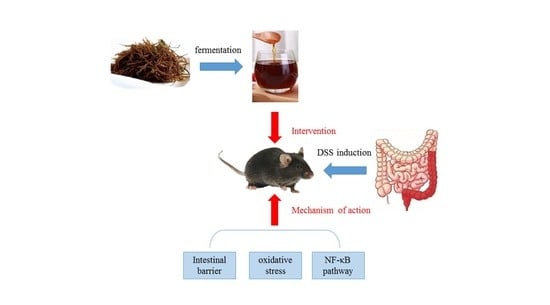
Fermented Sargassum fusiforme Mitigates Ulcerative Colitis in Mice by Regulating the Intestinal Barrier, Oxidative Stress, and the NF-κB Pathway
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of Fermented S. fusiforme
2.2. Determination of the Physicochemical Properties of Fermented S. fusiforme
2.3. Animal Experiments
2.3.1. Experimental Animals
2.3.2. Mice Colitis Model and Dietary Intervention
2.4. Disease Activity Index (DAI) in Mice
2.5. Histological Analysis
2.6. In Vivo Intestinal Permeability Measurement
2.7. Inflammatory Cytokine (IL-6, IL-8, TNF-α, and IL-1β) Assay and Western Blot Analysis
2.8. Nitric Oxide (NO) and Myeloperoxidase (MPO) Levels in Mice Serum
2.9. Total Superoxide Dismutase (SOD) Enzyme Activity in Colonic Tissue
2.10. The Colon Tissue for Malondialdehyde (MDA)
2.11. Measurement of Catalase (CAT) in Serum and Colonic Tissues
2.12. Detection of SCFA Content in Feces
2.13. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Fermented S. fusiforme
3.2. Fermented S. fusiforme Alleviated Symptoms of Colitis in Mice
3.3. Fermented S. fusiforme Attenuated Histological Damage Caused by DSS
3.4. Inhibition of Permeability Increase of Colonic Epithelium in Colitis by Fermented S. fusiforme
3.5. Protective Effect of Fermented S. fusiforme on DSS-Induced Tight Junction Proteins in Mice with Colitis
3.6. Fermented Sargassum Fusiforme Decreased Levels of Inflammatory Cytokines and the Inhibited NF-κB Pathway
3.7. Fermented S. fusiforme Reduced Oxidative Stress
3.8. Restoration of SCFA Production in the Cecum by Fermented S. fusiforme
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezzio, C.; Saibeni, S.; Variola, A.; Allocca, M.; Massari, A.; Gerardi, V.; Casini, V.; Ricci, C.; Zingone, F.; Amato, A.; et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 2020, 69, 1213–1217. [Google Scholar] [CrossRef]
- Shitrit, A.B.-G.; Grisaru-Granovsky, S.; Ben Ya’acov, A.; Goldin, E. Management of Inflammatory Bowel Disease During Pregnancy. Dig. Dis. Sci. 2016, 61, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sans, M.; Fiocchi, C. Inflammatory bowel disease: The role of environmental factors. Autoimmun. Rev. 2004, 3, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. New Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Willot, S.; Noble, A.; Deslandres, C. Methotrexate in the treatment of inflammatory bowel disease: An 8-year retrospective study in a Canadian pediatric IBD center. Inflamm. Bowel Dis. 2011, 17, 2521–2526. [Google Scholar] [CrossRef]
- Van Dieren, J.M.; Kuipers, E.J.; Samsom, J.N.; Nieuwenhuis, E.E.; van der Woude, J.C. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm. Bowel Dis. 2006, 12, 311–327. [Google Scholar] [CrossRef]
- Habens, F.; Srinivasan, N.; Oakley, F.; Mann, D.A.; Ganesan, A.; Packham, G. Novel sulfasalazine analogues with enhanced NF-kB inhibitory and apoptosis promoting activity. Apoptosis 2005, 10, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.; Kelleher, D. Glucocorticoid resistance in inflammatory bowel disease. J. Endocrinol. 2003, 178, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid therapy in inflammatory bowel disease: Mechanisms and clinical practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Otten, A.T.; Frijlink, H.W.; Dijkstra, G.; Kosterink, J.G. local tumor necrosis factor-α inhibition in inflammatory bowel disease. Pharmaceutics 2020, 12, 539. [Google Scholar] [CrossRef]
- Slevin, S.M.; Egan, L.J. New insights into the mechanisms of action of anti–tumor necrosis factor-α monoclonal antibodies in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 2909–2920. [Google Scholar] [CrossRef]
- Ali, U.A.; Martin, S.T.; Rao, A.D.; Kiran, R.P. Impact of Preoperative Immunosuppressive Agents on Postoperative Outcomes in Crohn’s Disease. Dis. Colon Rectum 2014, 57, 663–674. [Google Scholar] [CrossRef]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Pan, G.; Li, H.; Xu, Y.; Wu, W. Probiotic consortia and their metabolites ameliorate the symptoms of inflammatory bowel diseases in a colitis mouse model. Microbiol. Spectr. 2022, 10, e00657-22. [Google Scholar] [CrossRef]
- Lyu, S.; Pan, F.; Ge, H.; Yang, Q.; Duan, X.; Feng, M.; Liu, X.; Zhang, T.; Liu, J. Fermented egg-milk beverage alleviates dextran sulfate sodium-induced colitis in mice through the modulation of intestinal flora and short-chain fatty acids. Food Funct. 2022, 13, 702–715. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Li, X.; Wang, Y.; Huo, Z.; Lin, Y.; Li, J.; Yang, H.; Zhang, Z.; Yang, P. Fermented Astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis. Front. Nutr. 2022, 9, 1035912. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J.I.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.-B.; Li, Z.-R.; Wu, J.; Ou, Z.-R.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 2012, 50, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.-Y.; Chen, L.; Li, Q.-J.; Shen, Y.-Z.; Jin, L.; Zhang, X.; Chen, P.-C.; Wu, M.-J.; Choi, J.-I.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Woting, A.; Blaut, M. Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H mice. Nutrients 2018, 10, 685. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, J.; Zhang, L.; Qin, N.; Zhu, B.; Xia, X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food Funct. 2021, 12, 10121–10135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Teng, Y.; Zhang, S.; Zhu, B.; Xia, X. Gut microbiota mediates the anti-colitis effects of polysaccharides derived from Rhopilema esculentum Kishinouye in mice. Food Funct. 2023, 14, 1989–2007. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, B.; Liu, Z.; Wang, X.; Ai, C.; Gong, G.; Hu, M.; Huang, L.; Song, S. An arabinogalactan from Lycium barbarum attenuates DSS-induced chronic colitis in C57BL/6J mice associated with the modulation of intestinal barrier function and gut microbiota. Food Funct. 2021, 12, 9829–9843. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Chan, J.C.; Wang, X.; Zhao, C.; Xu, Y.; Xiong, W.; Chung, W.C.; Liang, F.; Wang, X. Chinese herbal medicines in the treatment of ulcerative colitis: A review. Chin. Med. 2022, 17, 43. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11. [Google Scholar] [CrossRef]
- Günzel, D.; Fromm, M. Claudins and Other Tight Junction Proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Murata, Y.; Ishiguro, Y.; Itoh, J.; Munakata, A.; Yoshida, Y. The role of proinflammatory and immunoregulatory cytokines in the pathogenesis of ulcerative colitis. J. Gastroenterol. 1995, 30, 56–60. [Google Scholar]
- Xiao, Y.-T.; Yan, W.-H.; Cao, Y.; Yan, J.-K.; Cai, W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine 2016, 83, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Luo, W.; Shen, Z.; Deng, M.; Li, X.; Tan, B.; Xiao, M.; Wu, S.; Yang, Z.; Zhu, C.; Tian, L.; et al. Roseburia intestinalis supernatant ameliorates colitis induced in mice by regulating the immune response. Mol. Med. Rep. 2019, 20, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Martín, R.; Torres-Maravilla, E.; Chadi, S.; González-Dávila, P.; Sokol, H.; Langella, P.; Chain, F.; Bermúdez-Humarán, L.G. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 2020, 12, 1826748. [Google Scholar] [CrossRef]









| Score | Weight Loss (%) | Stool Consistency | Occult/Gross Bleeding |
|---|---|---|---|
| 0 | None | Normal | Negative |
| 1 | 1–5 | Loose stool | Positive |
| 2 | 5–10 | ||
| 3 | 10–20 | ||
| 4 | >20 | Diarrhea | Gross bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Cao, Y.; Wang, Z.; Liu, H.; Teng, Y.; Li, G.; Liu, J.; Xia, X. Fermented Sargassum fusiforme Mitigates Ulcerative Colitis in Mice by Regulating the Intestinal Barrier, Oxidative Stress, and the NF-κB Pathway. Foods 2023, 12, 1928. https://doi.org/10.3390/foods12101928
Zhang S, Cao Y, Wang Z, Liu H, Teng Y, Li G, Liu J, Xia X. Fermented Sargassum fusiforme Mitigates Ulcerative Colitis in Mice by Regulating the Intestinal Barrier, Oxidative Stress, and the NF-κB Pathway. Foods. 2023; 12(10):1928. https://doi.org/10.3390/foods12101928
Chicago/Turabian StyleZhang, Siteng, Yu Cao, Zixuan Wang, Huanhuan Liu, Yue Teng, Guopeng Li, Jiaxiu Liu, and Xiaodong Xia. 2023. "Fermented Sargassum fusiforme Mitigates Ulcerative Colitis in Mice by Regulating the Intestinal Barrier, Oxidative Stress, and the NF-κB Pathway" Foods 12, no. 10: 1928. https://doi.org/10.3390/foods12101928