Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites
Abstract
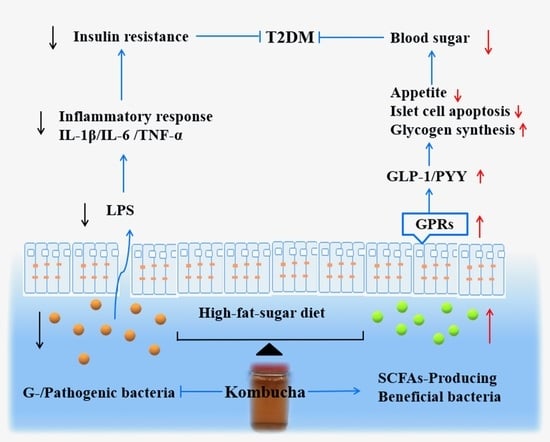
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Tea Soup and Kombucha
2.3. Components Analysis
2.4. Animal Experimental Design and Sample Collection
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Determination of Organ Index and Biochemical Indexes
2.7. Histopathological Evaluation
2.8. Gut Microbiota Analysis
2.9. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.10. The Short-Chain Fatty Acid (SCFA) in Fecal Samples
2.11. Statistical Analysis
3. Results and Discussion
3.1. The Fermentation Provided Kombucha with Different Components Compared with Tea Soup
3.2. Effects of Kombucha on Fasting Blood Glucose, Food Intake, Body Weight, Glucose Tolerance, Insulin and Glycogen Contents in T2DM Mice
3.3. Kombucha Improved the Damaged Liver and Islet Tissue in T2DM Mice
3.4. Kombucha Changed the Structure of the Gut Microbiota in T2DM Mice
3.5. Kombucha Regulated the Intestinal Metabolites in T2DM Mice
3.6. Kombucha Improved the Intestinal Permeability in T2DM Mice
3.7. Correlation Analysis of Gut Microbiota and Biochemical Indicators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ma, Q.T.; Li, Y.Q.; Li, P.F.; Wang, M.; Wang, J.K.; Tang, Z.Y. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Morris, M.R.; Ludwar, B.C.; Swingle, E.; Shubrook, J.H. A new method to assess asymmetry in fingerprints could be used as an early indicator of type 2 diabetes mellitu. J. Diabetes Sci Technol. 2016, 10, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.M.; Zhang, L.; Qing, Y.; Zhang, J.C. Glycolipid metabolism and metagenomic analysis of the therapeutic effect of a phenolics--rich extract from noni fruit on type 2 diabetic mice. J. Agric Food Chem. 2022. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhang, M.N.; Wu, T.C.; Xu, M.Y.; Cai, H.N.; Zhang, Z.S. Effects of d-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J. Agric Food Chem. 2015, 63, 19–26. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.X.; Shi, F.F. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 15315, 1231–1240. [Google Scholar] [CrossRef]
- David, A.C.; Sonia, R.M.; Elvira, L.O. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020, 132, 109058. [Google Scholar]
- Zhang, B.W.; Sun, W.L.; Yu, N.; Sun, J.; Yu, X.X.; Li, X. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Suda, W. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Anhê, F.F.; Jensen, B.A.H.; Varin, T.V. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2020, 104, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.W.; Wang, J.H.; Wang, J.F.; Yang, H.C.; Yan, X.J.; Su, L.J. Fucoidan from Acaudina molpadioides improves insulin resistance by altering gut microbiota dysfunction. J. Funct. Foods 2019, 57, 59–67. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.M.; Dinareer, D.; Wu, Y.C.; Sun, X. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharm. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Dipeeka, K.; Mandaliya; Sriram, S. Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes. Pancreatology 2019, 19, 280–284. [Google Scholar]
- Mindani, I.; Watawana; Nilakshi, J.; Viduranga, Y. Retracted: Application of the kombucha ‘tea fungus’ for the enhancement of antioxidant and starch hydrolase inhibitory properties of ten herbal teas. Food Chem. 2016, 194, 304–311. [Google Scholar]
- Pugliero, S.; Lima, D.Y.; Rodrigues, M.A.; Rogero, M. Kefir reduces nitrosative stress and upregulates Nrf2 in the kidney of diabetic rats. Int. Dairy J. 2021, 114, 104909. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N.; Kilin, A.K.; Karakaya, S. Vegetable and fermented vegetable juices containing germinated seeds and sprouts of lentil and cowpea. Food Chem. 2014, 156, 289–295. [Google Scholar] [CrossRef]
- Tu, C.H.; Tang, S.J.; Fidelis, A.; Hu, W.X. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 181–189. [Google Scholar] [CrossRef]
- Semantee, B.; Ratan, G.; Parames, C.S. Effect of Kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar]
- Li, Y.; Rahman, S.U.; Huang, Y.Y.; Zhang, Y.F.; Ming, P.F. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Wang, M.J.; Chen, G.J.; Chen, D.; Hong, Y.; Sun, Y. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. Int. J. Biol. Macromol. 2019, 1401, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.K.; Zeng, F.; Wu, L.X.; Wan, X.Z.; Chen, Y.F. Fermented carrot juice attenuates type 2 diabetes. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.Y.; Luo, J.Y.; Bao, Y.H. Effects of Polygonatum sibiricum saponin on hyperglycemia, gut microbiota composition and metabolic profiles in type 2 diabetes mice. Biomed.Pharmacother. 2021, 143, 112155. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.D.; Dai, Y.Q.; Wu, H.; Liu, X.L.; Wang, Y. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Hemmati, M.; Karamian, M.; Malekaneh, M. Anti-atherogenic potential of natural honey: Anti-diabetic and antioxidant approaches. J. Pharm. Pharmacol. 2015, 3, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Y.; Xu, X.Y.; Gan, R.Y.; Sun, Q.C.; Meng, J.M. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 2019, 8, 440. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Watanabe, K.; Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Wang, R.M.; Yao, L.L.; Lin, X.; Hu, X.P.; Wang, L. Exploring the potential mechanism of Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic rich extract on ameliorating nonalcoholic fatty liver disease by integration of transcriptomics and metabolomics profiling. Food Res. Int. 2022, 151, 110824. [Google Scholar] [CrossRef]
- Ding, Q.Z.; Zhang, B.; Zheng, W.; Chen, X.J.; Zhang, J. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in rats induced by streptozotocin and high-fat, high-sugar diet. Biomed. Pharm. 2019, 118, 109262. [Google Scholar] [CrossRef]
- Liu. G., M.; Liang, L.; Yu, G.Y.; Li, Q.H. Pumpkin polysaccharide modififies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Liu, M.M.; Ma, R.H.; Ni, Z.J.; Thakur, K.; Cespedes-Acuna, C.L.; Jiang, L.; Wei, Z.J. Apigenin 7-O-glucoside promotes cell apoptosis through the PTEN/PI3K/AKT pathway and inhibits cell migration in cervical cancer HeLa cells. Food Chem. Toxicol. 2020, 146, 111843. [Google Scholar] [CrossRef] [PubMed]
- Sinir, G.Ö.; Tamer, C.E.; Suna, S.; Senem, S. Kombucha tea: A promising fermented functional beverage. Fermented Beverages 2019, 32, 401–432. [Google Scholar]
- Viesser, J.A.; Pereira, G.M.; Rogez, H.; Azevedo, V.; Brenig, B. Co-culturing fructophilic lactic acid bacteria and yeast enhanced sugar metabolism and aroma formation during cocoa beans fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.R.; Neto, R.O.; D’Almeida, C.T.S.; Nascimento, T.P. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T. Impact of fermentation conditions on the production of bioactive compounds with anticancer anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Filippis, F.D.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during Kombucha tea fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef]
- Zhuo, X.H.; Cohen, C.M.; Chen, J.L.; Chodick, G. Validating the UK prospective diabetes study outcome model 2 using data of 94,946 Israeli patients with type 2 diabetes. J. Diabetes Complicat. 2021, 12, 108086. [Google Scholar] [CrossRef]
- Feng, J.H.; Zhao, H.; Du, M.Z.; Wu, X.P. The effect of apelin-13 on pancreatic islet beta cell mass and myocardial fatty acid and glucose metabolism of experimental type 2 diabetic rats. Peptides 2019, 114, 1–7. [Google Scholar] [CrossRef]
- Gheibi, S.; Bakhtiarzadeh, F.; Jeddi, S.; Zardooz, H. Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric. Oxide 2017, 64, 39–51. [Google Scholar] [CrossRef]
- Leylabadlo, H.E.; Sanaie, S.; Heravi, F.S. From role of gut microbiota to microbial based therapies in type 2-diabetes. Infect. Genet. Evol. 2020, 81, 104268. [Google Scholar] [CrossRef]
- Han, S.; Luo, Y.; Hu, Z.M.; Qin, D.D.; Luo, F.J. Targeting gut microbiota in type 2 diabetes mellitus: Potential roles of dietary flavonoids. Food Biosci. 2022, 45, 101500. [Google Scholar] [CrossRef]
- Yan, H.L.; Lu, J.M.; Wang, Y.F.; Gu, W.; Yang, X.X.; Yu, J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine 2017, 26, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sibusiso, L.; Hou, L.F.; Jiang, H.J.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.Y.; Wang, Y.H.; Lin, Y.; Li, E.H.; Zhang, X.Y. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Natividad, J.M.; Pinto-Sanchez, M.I.; Galipeau, H.J.; Jury, J. Ecobiotherapy rich in Firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm. Bowel Dis. 2015, 8, 1. [Google Scholar] [CrossRef]
- Oh, J.H.; Alexander, L.M.; Pan, M.C.; Schueler, K.L. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont lactobacillus reuteri. Cell Host Microbe 2019, 25, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.Z.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 04403. [Google Scholar] [CrossRef]
- Vreugdenhil, A.C.E.; Rousseau, C.H.; Hartung, T.; Greve, J.W.M. Lpopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 2003, 170, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.C.; Weng, H.B.; Ling, L.J. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int. J. Biol. Macromol. 2019, 132, 1001–1011. [Google Scholar] [CrossRef]
- Qin, J.J.; Li, Y.R.; Cai, Z.M.; Li, S.H.; Zhu, J.F. A metagenome-wide association study of gut microbiota in type II diabete. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Zhao, A.Z. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowd, V.; Karim, N.; Xie, L.H.; Chen, E. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Tech. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Li, K.K.; Tian, P.J.; Wang, S.D. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J. Funct. Foods 2017, 38, 561–570. [Google Scholar] [CrossRef]
- Yu, W.; Gao, D.P.; Jin, W.; WANG, Z.J. Intestinal flora dysbiosis aggravates cognitive dysfunction associated with neuroinflammation in heart failure. J. Card. Fail. 2020, 26, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Guo, L.X.; Hu, W.H. Polysaccharide from tuberous roots of Ophiopogon japonicus regulates gut microbiota and its metabolites during alleviation of high-fat diet-induced type-2 diabetes in mice. J. Funct. Foods 2019, 63, 103593. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, B.B.; Hu, A.L.; Li, H.; Liu, J.; Zhang, F. Protective role of cinnabar and realgar in Hua-Feng-Dan against LPS plus rotenone-induced neurotoxicity and disturbance of gut microbiota in rats. J. Ethnopharmacol. 2020, 247, 112299. [Google Scholar] [CrossRef]
- Chen, R.Z.; Wu, P.; Cai, Z.; Tang, L.; Ye, L. The combination of Puerariae Lobatae Radix and Chuanxiong Rhizoma enhanced the absorption and pharmacokinetics of puerarin by modulating the intestinal barrier and influenced gut microbiota. J. Funct. Foods 2018, 47, 72–82. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Mucida, D. Neuro-immune interactions at barrier surfaces. Cell 2016, 165, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Ma, B.Q.; Zhao, Y.Z.; Li, Q.R.; Li, Q.R. Lipopolysaccharide-induced bacterial translocation is intestine site-specific and associates with intestinal mucosal inflammation. Inflammation 2012, 35, 1880–1888. [Google Scholar] [CrossRef]
- Gu, J.F.; Shu, L.S.; Guo, J.M. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J. Funct. Foods 2017, 36, 362–374. [Google Scholar] [CrossRef]







| Content (mg/mL) | KT | TS |
|---|---|---|
| Total acid | 8.6 ± 0.87 | ND ** |
| Total sugar | 18.73 ± 2.87 | 158 ± 1.67 ** |
| Total phenol acid | 1.39 ± 0.15 | 0.09 ± 0.02 ** |
| Formic acid | 0.25 ± 0.06 | ND ** |
| Acetic acid | 3.03 ± 0.81 | ND ** |
| Butyric acid | 0.42 ± 0.11 | ND ** |
| Citric acid | 0.67 ± 0.21 | ND ** |
| Lactic acid | 0.41 ± 0.13 | ND ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods 2022, 11, 754. https://doi.org/10.3390/foods11050754
Xu S, Wang Y, Wang J, Geng W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods. 2022; 11(5):754. https://doi.org/10.3390/foods11050754
Chicago/Turabian StyleXu, Suyun, Yanping Wang, Jinju Wang, and Weitao Geng. 2022. "Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites" Foods 11, no. 5: 754. https://doi.org/10.3390/foods11050754