Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Supplementation and RFV Preparation
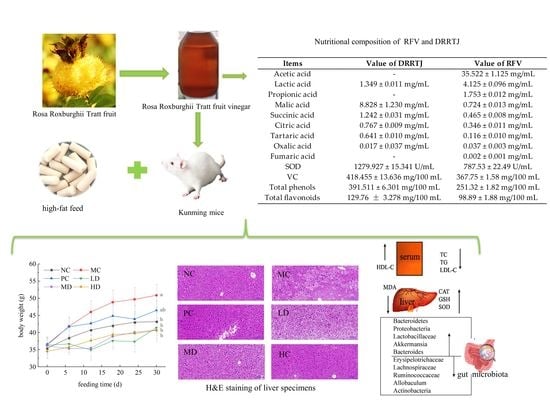
2.2. Measurement of Chemical Functional Components
2.3. Animal Experiment Design
2.4. Organ Index of Mice Were Measured
2.5. Serum and Liver Biochemical Analysis
2.6. Histological Analysis
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Bioactive Compounds in RFV
3.2. Anti-Obesity Potential of RFV
3.3. Potential Preventing Dyslipidemia of RFV
3.4. Hepatic Antioxidant Power of RFV
3.5. Histological Trait of Mice Liver
3.6. Effects of RFV on Gut Microbiota in Obese Mice
3.7. Correlation between Obesity-Related Parameters and Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.T.; Lv, M.J.; An, J.Y. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Yang, H.; Zhao, Y.; Xu, L.; Zhao, L.; Wang, Y.; Liao, X. Chemical characterization and comparison of two chestnut rose cultivars from different regions. Food Chem. 2020, 323, 126806. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107, 166–174. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Ma, N.; Zhang, X.; Liu, M.; Fu, W. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide fromRosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabeticdb/dbMice. J. Agr. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, S.; Tang, L.; Zhang, M.; Yuan, M.; Wang, P.; Gao, X. Integrative analysis of fecal metabolome and gut microbiota in high-fat diet-induced hyperlipidemic rats treated with Rosa Roxburghii Tratt juice. J. Funct. Foods 2022, 90, 104978. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Deng, L.; Zhao, M. Probiotic-fermented blueberry juice prevents obesity and hyperglycemia in high fat diet-fed mice in association with modulating the gut microbiota. Food Funct. 2020, 10, 9192–9207. [Google Scholar] [CrossRef]
- Ya-Fang, S.; Heng, C.; Yi-Long, M.; Chen, Z.; Fei, M. Effect of lactic acid bacteria fermentation on tannins removal in Xuan Mugua fruits. Food Chem. 2019, 274, 118–122. [Google Scholar]
- Chaturvedi, S.; Chakraborty, S. Optimization of extraction process for legume-based synbiotic beverages, followed by their characterization and impact on antinutrients. Int. J. Gastron. Food Sci. 2022, 28, 100506. [Google Scholar] [CrossRef]
- CanaviriPaz, P.; Oscarsson, E.; Kjellström, A.; Olsson, H.; Jois, C. Effects on Microbiota Composition after Consumption of Quinoa Beverage Fermented by a Novel Xylose-Metabolizing L. plantarum Strain. Nutrients 2021, 13, 3318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Cao, J.; Jin, H.; Shan, Y.; Fang, J.; Liu, F. Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice. Food Funct. 2021, 12, 9151–9164. [Google Scholar] [CrossRef] [PubMed]
- Nevin, Ş.; Başar, G.B.; Ceyhun, S.A. Health benefits of fermented foods. Crit. Rev. Food Sci. 2019, 59, 506–527. [Google Scholar]
- Chen, X.; Yuan, M.; Wang, Y.; Zhou, Y.; Sun, X. Influence of fermentation with different lactic acid bacteria andin vitro digestion on the change of phenolic compounds in fermented kiwifruit pulps. Int. J. Food Sci. Technol. 2021, 5, 2670–2679. [Google Scholar]
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Boo, S.Y. Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat-diet-induced obese mice. Sci Rep. 2017, 7, 6664. [Google Scholar] [CrossRef] [Green Version]
- Anuar, S.; Azrina, A.; Amin, I. Therapeutic effects of vinegar: A review. Curr. Opin. Food Sci. 2016, 8, 56–61. [Google Scholar]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Mohamad, N.E.; Yeap, S.K.; Ky, H.; Ho, W.Y.; Boo, S.Y.; Chua, J.; Beh, B.K.; Sharifuddin, S.A.; Long, K.; Alitheen, N.B. Dietary coconut water vinegar for improvement of obesity-associated inflammation in high-fat-diet-treated mice. Food Nutr. Res. 2017, 61, 1368322. [Google Scholar] [CrossRef] [Green Version]
- Hosoda, S.; Kawazoe, Y.; Shiba, T.; Numazawa, S.; Manabe, A. Anti-Obesity Effect of Ginkgo Vinegar, a Fermented Product of Ginkgo Seed Coat, in Mice Fed a High-Fat Diet and 3T3-L1 Preadipocyte Cells. Nutrients 2020, 12, 230. [Google Scholar] [CrossRef] [Green Version]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Du, M.; Mao, X. Milk fat globule membrane supplementation modulates the gut microbiota and attenuates metabolic endotoxemia in high-fat diet-fed mice. J. Funct. Foods 2018, 47, 56–65. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstructionvia leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.; Zhao, C.; Gan, R.; Li, H. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H. Biological Function of Acetic Acid-Improvement in Obesity and Glucose Tolerance by Acetic Acid in Type 2 Diabetic Rats. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S171–S175. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700485. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Li, Y.; Zhao, S.; Chen, C.; Ning, D. Alleviating effects of walnut green husk extract on disorders of lipid levels and gut bacteria flora in high fat diet-induced obesity rats. J. Funct. Foods 2019, 52, 576–586. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; Chen, H.; Zhang, H.; Hu, H.; Dai, X. Potato resistant starch inhibits diet-induced obesity by modifying the composition of intestinal microbiota and their metabolites in obese mice. Int. J. Biol. Macromol. 2021, 180, 458–469. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Li, X.; Xin, X.; Liu, D. Daily Supplementation with Fresh Angelica keiskei Juice Alleviates High-Fat Diet-Induced Obesity in Mice by Modulating Gut Microbiota Composition. Mol. Nutr. Food Res. 2019, 63, 1900248. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Teng, C.Y.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Garcinol Reduces Obesity in High-Fat-Diet-Fed Mice by Modulating Gut Microbiota Composition. Mol. Nutr. Food Res. 2019, 63, 1800390. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liu, J.; Zheng, S.; Liang, F. Mulberry leaves ameliorate obesity through enhancing brown adipose tissue activity and modulating gut microbiota. Food Funct. 2019, 10, 4771–4781. [Google Scholar] [CrossRef] [PubMed]
- Xinyao, J.; Yuehua, W.; Yang, L.; Yuxi, L. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2018, 64, 88–100. [Google Scholar]
- Scheithauer, T.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhu, Y.; Ma, Y.; Xiang, Q.; Shen, R.; Liu, Y. Oat products modulate the gut microbiota and produce anti-obesity effects in obese rats. J. Funct. Foods 2016, 25, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Ao, S.; Renyou, G.; Xiaoyu, X.; Qianqian, M.; Pangzhen, Z.; Huabin, L. Effects and mechanisms of edible and medicinal plants on obesity: An updated review. Crit. Rev. Food Sci. 2020, 61, 2061–2077. [Google Scholar]
- Mohamad, N.E.; Yeap, S.K.; Ky, H. Pineapple Vinegar Regulates Obesity-Related Genes and Alters the Gut Microbiota in High-Fat Diet (HFD) C57BL/6 Obese Mice. Evid. Based Compl. Alt. 2020, 2020, 1257962. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Ugajin, S.; Kaga, T. Vinegar Intake Reduces Body Weight, Body Fat Mass, and Serum Triglyceride Levels in Obese Japanese Subjects. Biosci. Biotechnol. Biochem. 2014, 73, 1837–1843. [Google Scholar] [CrossRef] [Green Version]
- Yañez, C.M.; Hernández, A.M.; Sandoval, A.M.; Domínguez, M.A.M.; Muñiz, S.A.Z.; Gómez, J.O.G. Prevalence of Blastocystis and its association with Firmicutes/Bacteroidetes ratio in clinically healthy and metabolically ill subjects. BMC Microbiol. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, S.; Wang, X.; Weng, Y.; Fan, X.; Sheng, H.; Zhu, X.; Lou, L.; Zhang, F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Yang, S.; Zhang, L.; Qiao, L.; Wang, L.; He, S.; Li, J.; Yang, N.; Yue, B.; Zhou, C. Comparative metagenomics analysis reveals how the diet shapes the gut microbiota in several small mammals. Ecol. Evol. 2022, 12, e8470. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jing, Y.; Li, Y.; Zhang, N.; Zhang, W.; Cao, Y. The differential modulatory effects of Eurotium cristatum on the gut microbiota of obese dogs and mice are associated with improvements in metabolic disturbances. Food Funct. 2021, 12, 12812–12825. [Google Scholar] [CrossRef]
- Cox, L.M.; Cho, I.; Young, S.A.; Anderson, W.H.K.; Waters, B.J.; Hung, S.C.; Gao, Z.; Mahana, D.; Bihan, M.; Alekseyenko, A.V.; et al. The nonfermentable dietary fiber hydroxypropyl methylcellulose modulates intestinal microbiota. FASEB J. 2012, 27, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; Bergen, M.V.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Ziętak, M.; Kovatcheva-Datchary, P.; Markiewicz, L.H.; Ståhlman, M.; Kozak, L.P.; Bäckhed, F. Altered Microbiota Contributes to Reduced Diet-Induced Obesity upon Cold Exposure. Cell Metab. 2016, 23, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Thomaz, F.S.; Tomsett, K.I.; Panchal, S.K. Wasabi supplementation alters the composition of the gut microbiota of diet-induced obese rats. J. Funct. Foods 2020, 67, 103868. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhu, L.; Liu, Z.; Ye, C.; Qin, S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep. 2020, 10, 978. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Qiao, X.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Li, Y.; Gao, H. Differences of gut microbiota composition in mice supplied with polysaccharides from γ-irradiated and non-irradiated Schizophyllum commune. Food Res. Int. 2022, 151, 110855. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Tan, M.; Chen, X. Punicic acid ameliorates obesity and liver steatosis by regulating gut microbiota composition in mice. Food Funct. 2021, 12, 7897–7908. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Jiang, J.; Shi, Z.; Wu, L.; Yan, J.; Chen, Z.; Luo, M.; Cui, D.; Xu, S.; Yan, M.; et al. Pure total flavonoids from citrus attenuate non-alcoholic steatohepatitis via regulating the gut microbiota and bile acid metabolism in mice. Biomed. Pharmacother. 2021, 135, 111183. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Jia, R.B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Xiong, L.; Zhao, L.; Guo, H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
- Yasuma, T.; Toda, M.; Abdel-Hamid, A.M.; D Alessandro-Gabazza, C.; Kobayashi, T.; Nishihama, K.; D Alessandro, V.F.; Pereira, G.V.; Mackie, R.I.; Gabazza, E.C.; et al. Degradation Products of Complex Arabinoxylans by Bacteroides intestinalis Enhance the Host Immune Response. Microorganisms 2021, 9, 1126. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl. Microbiol. Biot. 2019, 103, 2353–2365. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in Gut Microbiota in Rats Fed a High Fat Diet Correlate with Obesity-Associated Metabolic Parameters. PLoS ONE 2015, 10, e126931. [Google Scholar] [CrossRef]









| Items | Value of DRRTJ | Value of RFV |
|---|---|---|
| Acetic acid | - | 35.522 ± 1.125 mg/mL |
| Lactic acid | 1.349 ± 0.011 mg/mL | 4.125 ± 0.096 mg/mL |
| Propionic acid | - | 1.753 ± 0.012 mg/mL |
| Malic acid | 8.828 ± 1.230 mg/mL | 0.724 ± 0.013 mg/mL |
| Succinic acid | 1.242 ± 0.031 mg/mL | 0.465 ± 0.008 mg/mL |
| Citric acid | 0.767 ± 0.009 mg/mL | 0.346 ± 0.011 mg/mL |
| Tartaric acid | 0.641 ± 0.010 mg/mL | 0.116 ± 0.010 mg/mL |
| Oxalic acid | 0.017 ± 0.037 mg/mL | 0.037 ± 0.003 mg/mL |
| Fumaric acid | - | 0.002 ± 0.001 mg/mL |
| SOD | 1279.927 ± 15.341 U/mL | 787.53 ± 22.49 U/mL |
| VC | 418.455 ± 13.636 mg/100 mL | 367.75 ± 1.58 mg/100 mL |
| Total phenols | 391.511 ± 6.301 mg/100 mL | 251.32 ± 1.82 mg/100 mL |
| Total flavonoids | 129.76 ± 3.278 mg/100 mL | 98.89 ± 1.88 mg/100 mL |
| Group | Cardiac Index | Liver Index | Kidney Index | Spleen Index |
|---|---|---|---|---|
| NC | 0.71 ± 0.05 b | 4.42 ± 0.26 ab | 0.27 ± 0.06 b | 1.66 ± 0.16 a |
| MC | 0.60 ± 0.08 d | 4.93 ± 0.17 a | 0.30 ± 0.05 b | 1.40 ± 0.08 bc |
| PC | 0.79 ± 0.06 a | 4.35 ± 0.47 ab | 0.28± 0.06 b | 1.54 ± 0.14 ab |
| LD | 0.69 ± 0.05 b | 4.34 ± 0.72 ab | 0.27± 0.08 b | 1.40 ± 0.16 bc |
| MD | 0.65 ± 0.08 c | 4.34 ± 0.89 ab | 0.39 ± 0.07 a | 1.40 ± 0.16 bc |
| HD | 0.71 ± 0.05 b | 4.21 ± 0.63 b | 0.32 ± 0.03 ab | 1.31 ± 0.17 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, J.; Zhang, Y.; Shi, Y.; Feng, D.; Zuo, Y.; Hu, P. Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice. Foods 2022, 11, 4108. https://doi.org/10.3390/foods11244108
Li J, Zhang J, Zhang Y, Shi Y, Feng D, Zuo Y, Hu P. Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice. Foods. 2022; 11(24):4108. https://doi.org/10.3390/foods11244108
Chicago/Turabian StyleLi, Jiuchang, Jun Zhang, Yulong Zhang, Yuanyuan Shi, Dandan Feng, Yunyang Zuo, and Ping Hu. 2022. "Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice" Foods 11, no. 24: 4108. https://doi.org/10.3390/foods11244108