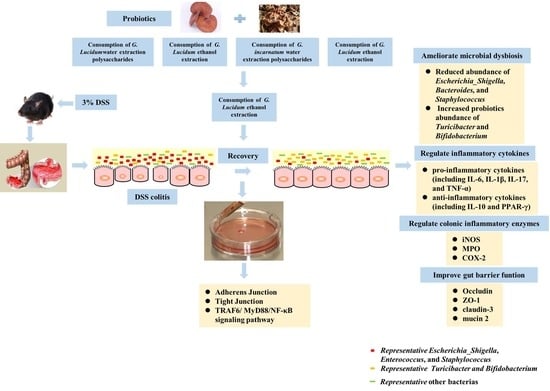
Ganoderma lucidum Ethanol Extraction Promotes Dextran Sulphate Sodium Induced Colitis Recovery and Modulation in Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the G. lucidum Extracts
2.2. DSS-Induced Animal Study
2.3. Histopathology
2.4. ELISA Measurement
2.5. Fecal Genomic DNA Extraction and 16S rRNA Sequencing
2.6. Caco-2 Cell Experiment
2.7. Measurement of Cytokines using Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Influence of G. lucidum and G. incarnatum Extract Administration on the Recovery of DSS-Induced Colitis
3.2. Influence of G. lucidum and G. incarnatum Extract Administration on DSS-Induced Colonic Tissue Damage and Regulation of Inflammatory Enzymes
3.3. Effects of G. lucidum Extract Administration on the Regulation of Inflammatory Cytokines
3.4. Effects of G. lucidum Extract Administration on the Protection of Intestinal Barrier
3.5. Intestinal Microbiota Modulation by GLE Administration
3.6. GLE Improved Intestinal Barrier Function and Inhibited TRAF6/MyD88/NF-κB Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malinowski, B.; Wiciński, M.; Sokołowska, M.M.; Hill, N.A.; Szambelan, M. The rundown of dietary supplements and their effects on inflammatory bowel disease—A review. Nutrients 2020, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Yangyang, R.Y.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar]
- Barbalho, S.M.; de Alvares Goulart, R.; Quesada, K.; Bechara, M.D.; de Carvalho, A.d.C.A. Inflammatory bowel disease: Can omega-3 fatty acids really help? Ann. Gastroenterol. 2016, 29, 37. [Google Scholar] [PubMed]
- Farrokhyar, F.; Marshall, J.K.; Easterbrook, B.; Irvine, J.E. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: Prevalence and impact on health. Inflamm. Bowel Dis. 2006, 12, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; Ford, A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.A.; Frank, D.N.; Pace, N.R.; Gordon, J.I. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 2008, 3, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.; Harris, P.J.; Ferguson, L.R. Potential benefits of dietary fibre intervention in inflammatory bowel disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar] [CrossRef] [Green Version]
- Rapozo, D.C.; Bernardazzi, C.; de Souza, H.S.P. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 2017, 23, 2124. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.-M.; Xu, H.-Y.; Xu, G.-H.; Shi, J.-S.; Xu, Z.-H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Dramae, A.; Boonyuen, N.; Veeranondha, S.; Rachtawee, P.; Pittayakhajonwut, P. Antimicrobial activity of illudalane and alliacane sesquiterpenes from the mushroom Gloeostereum incarnatum BCC41461. Phytochem. Lett. 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Li, M.; Yu, L.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q.; Tian, F. Role of dietary edible mushrooms in the modulation of gut microbiota. Phytochem. Lett. 2021, 83, 104538. [Google Scholar] [CrossRef]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2005, 2005, 63–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, Q.; Qu, Y.; Wang, M.; Li, L.; Liu, Y.; Li, Y. The investigation of immunomodulatory activities of Gloeostereum incaratum polysaccharides in cyclophosphamide-induced immunosuppression mice. Exp. Ther. Med. 2018, 15, 3633–3638. [Google Scholar]
- Zhang, Z.; Taylor, L.; Shommu, N.; Ghosh, S.; Reimer, R.; Panaccione, R.; Kaur, S.; Hyun, J.E.; Cai, C.; Deehan, E.C. A diversified dietary pattern is associated with a balanced gut microbial composition of Faecalibacterium and Escherichia/Shigella in patients with Crohn’s disease in remission. Crohns Colitis 2020, 14, 1547–1557. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrne, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar]
- Asai, R.; Mitsuhashi, S.; Shigetomi, K.; Miyamoto, T.; Ubukata, M. Absolute configurations of (−)-hirsutanol A and (−)-hirsutanol C produced by Gloeostereum incarnatum. J. Antibiot. 2011, 64, 693–696. [Google Scholar]
- Wang, X.; Peng, J.; Sun, L.; Bonito, G.; Wang, J.; Cui, W.; Fu, Y.; Li, Y. Genome sequencing illustrates the genetic basis of the pharmacological properties of Gloeostereum incarnatum. Genes 2019, 10, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Fan, J.; Liu, Y.; Guo, W.; Cao, H.; Xiao, J.; Liu, B. Hepatoprotective activity of Ganoderma lucidum triterpenoids in alcohol-induced liver injury in mice, an iTRAQ-based proteomic analysis. Food Chem. 2019, 271, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Pan, Y.Y.; Li, L.; Li, T.T.; Liu, B.; Lv, X.C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, A.O.; Awosanya, S.A.; Adeyi, O.E.; James, A.S.; Adenipekun, C.O. Ganoderma lucidum ethanol extract abrogates metabolic syndrome in rats: In vivo evaluation of hypoglycemic, hypolipidemic, hypotensive and antioxidant properties. Obes. Med. 2021, 22, 100320. [Google Scholar] [CrossRef]
- Hu, R.; Guo, W.; Huang, Z.; Li, L.; Liu, B.; Lv, X. Extracts of Ganoderma lucidum attenuate lipid metabolism and modulate gut microbiota in high-fat diet fed rats. J. Funct. Foods 2018, 46, 403–412. [Google Scholar] [CrossRef]
- Li, M.; Yu, L.; Zhai, Q.; Liu, B.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Ganoderma applanatum polysaccharides and ethanol extracts promote the recovery of colitis through intestinal barrier protection and gut microbiota modulations. Food Funct. 2022, 13, 688–701. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Extraction, structural analysis and antioxidant activity of aloe polysaccharide. J. Mol. Struct. 2023, 1273, 134379. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Tang, B.; Wang, F.-C.; Tang, L.; Lei, Y.-Y.; Luo, Y.; Huang, S.-J.; Yang, M.; Wu, L.-Y.; Wang, W. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225. [Google Scholar] [CrossRef]
- Cui, G.; Martin, R.C.; Jin, H.; Liu, X.; Pandit, H.; Zhao, H.; Cai, L.; Zhang, P.; Li, W.; Li, Y. Up-regulation of FGF15/19 signaling promotes hepatocellular carcinoma in the background of fatty liver. J. Exp. Clin. Cancer Res. 2018, 37, 136. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Ding, J.; Zhang, H.; Shen, J.; Hao, Y.; Zhang, X.; Qi, W.; Luo, X.; Zhang, T.; Wang, N. Lactobacillus casei LH23 modulates the immune response and ameliorates DSS-induced colitis via suppressing JNK/p-38 signal pathways and enhancing histone H3K9 acetylation. Food Funct. 2020, 11, 5473–5485. [Google Scholar] [CrossRef]
- Romero-Nava, R.; Alarcón-Aguilar, F.J.; Giacoman-Martínez, A.; Blancas-Flores, G.; Aguayo-Cerón, K.A.; Ballinas-Verdugo, M.A.; Sánchez-Muñoz, F.; Huang, F.; Villafaña-Rauda, S.; Almanza-Pérez, J.C. Glycine is a competitive antagonist of the TNF receptor mediating the expression of inflammatory cytokines in 3T3-L1 adipocytes. Inflamm. Res. 2021, 70, 605–618. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, H.; Li, W.; Xie, M. Current development of polysaccharides from Ganoderma: Isolation, structure and bioactivities. Bioact. Carbohydr. Diet. Fibre 2013, 1, 10–20. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Y.; Stanton, C.; Paul Ross, R.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Alleviation effects of Bifidobacterium breve on DSS-induced colitis depends on intestinal tract barrier maintenance and gut microbiota modulation. Eur. J. Nutr. 2021, 60, 369–387. [Google Scholar] [CrossRef]
- Jin, B.-R.; Chung, K.-S.; Cheon, S.-Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.-J.; An, H.-J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Bhatia, P.; Alam, O.; Naim, M.J.; Nawaz, F.; Sheikh, A.A.; Jha, M. Recent advancement in the discovery and development of COX-2 inhibitors: Insight into biological activities and SAR studies (2008–2019). Bioorg. Chem. 2019, 89, 103007. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Tsung, A.; Huang, H.; Du, Q.; Yang, M.; Deng, M.; Xiong, S.; Wang, X.; Zhang, L. iNOS promotes CD24+ CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E10127–E10136. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.; Wang, C.; Zeng, Z.; Ren, Y.; Li, D.; Song, J.-L. Ameliorative effect of sinapic acid on dextran sodium sulfate-(DSS-) induced ulcerative colitis in Kunming (KM) mice. Oxid. Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Dokladny, K.; Smith, M.A.; Ye, D.; Kaza, A.; Watterson, D.M.; Ma, T.Y. Mechanism of interleukin-1β induced-increase in mouse intestinal permeability in vivo. J. Interferon Cytokine Res. 2012, 32, 474–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Daryani, N.; Saghazadeh, A.; Moossavi, S.; Sadr, M.; Shahkarami, S.; Soltani, S.; Farhadi, E.; Rezaei, N. Interleukin-4 and interleukin-10 gene polymorphisms in patients with inflammatory bowel disease. Immunol. Investig. 2017, 46, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Pawłowska, B.; Sobieszczańska, B.M. Intestinal epithelial barrier: The target for pathogenic Escherichia coli. Adv. Clin. Exp. Med. 2017, 26, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokine and gut microbiota modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef]
- Ni, J.; Shen, T.-C.D.; Chen, E.Z.; Bittinger, K.; Bailey, A.; Roggiani, M.; Sirota-Madi, A.; Friedman, E.S.; Chau, L.; Lin, A. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci. Transl. Med. 2017, 9, eaah6888. [Google Scholar] [CrossRef] [Green Version]
- Lagerqvist, N.; Löf, E.; Enkirch, T.; Nilsson, P.; Roth, A.; Jernberg, C. Outbreak of gastroenteritis highlighting the diagnostic and epidemiological challenges of enteroinvasive Escherichia coli, County of Halland, Sweden, November 2017. Eurosurveillance 2020, 25, 1900466. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Chen, G.; Chen, D.; Ye, H.; Sun, Y.; Zeng, X.; Liu, Z. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. Int. J. Biol. Macromol. 2019, 140, 858–870. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Zbinden, R.; Altwegg, M. Turicibacter sanguinis gen. nov., sp. nov., a novel anaerobic, Gram-positive bacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 1263–1266. [Google Scholar]
- Jung, M.-J.; Lee, J.; Shin, N.-R.; Kim, M.-S.; Hyun, D.-W.; Yun, J.-H.; Kim, P.S.; Whon, T.W.; Bae, J.-W. Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 2016, 6, 30887. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Tian, Z.; Li, L.; Zeng, Z.; Chen, M.; Xiong, L. Fecal microbiota alterations associated with diarrhea-predominant irritable bowel syndrome. Front. Microbiol. 2018, 9, 1600. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, J.M.; Margolis, K.G. Building community in the gut: A role for mucosal serotonin. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 6–8. [Google Scholar] [CrossRef]
- Zhong, Y.; Nyman, M.; Fåk, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef]
- Yan, S.; Yang, B.; Zhao, J.; Zhao, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019, 10, 1595–1608. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.; Lee, J.H.; Kim, J.H.; Che, X.; Ma, H.W.; Seo, D.H.; Kim, T.I.; Kim, W.H.; Kim, S.W. Lactobacillus acidophilus suppresses intestinal inflammation by inhibiting endoplasmic reticulum stress. J. Gastroenterol. Hepatol. 2019, 34, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-S.; Choi, J.W.; Jhun, J.; Kwon, J.Y.; Lee, B.-I.; Yang, C.W.; Park, S.-H.; Cho, M.-L. Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and Treg cell balance and fibrosis development. J. Med. Food 2018, 21, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Liu, Y.; Song, Y.; Gao, Y.; Zhao, F.; Luo, Y.; Qian, F.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020, 11, 5205–5222. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Y.; Wang, G.; Yang, Y.; Song, X.; Xiong, Z.; Zhang, H.; Lai, P.; Wang, S.; Ai, L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 2020, 67, 103854. [Google Scholar] [CrossRef]







| Score | Weight Loss | Stool Consistency | Blood Stool |
|---|---|---|---|
| 0 | No loss | Normal | No blood |
| 1 | 1–5% | Loose stool | |
| 2 | 5–10% | Watery diarrhea | Presence of blood |
| 3 | 10–20% | Slimy diarrhea, little blood | |
| 4 | >20% | Severe watery diarrhea with blood | Gross bleeding |
| Gene | Sequence (5’ to 3’) | |
|---|---|---|
| Forward | Reverse | |
| GAPDH | GACAAGCTTCCCGTTCTCAG | GAGTCAACGGATTTGGTCGT |
| IL-6 | ACCAGAGGAAATTTTCAATAGGC | TGATGCACTTGCAGAAAACA |
| TNF-α | TGCCTATGTCTCAGCCTCTTC | GGTCTGGGCCATAGAACTGA |
| IL-1β | ACCTTCCAGGATGAGGACATGA | CTAATGGGAACGTCACAC ACCA |
| IL-17 | CTCCAGAAGGCCCTCAGACTAC | GGGTCTTCATTGCGGTGG |
| PPAR-γ | CTGCTCAAGTATGGTGTCCATGA | TGAGATGAGGACTCCATCTTT ATTCA |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| β-actin | CCTTCCCTCCTCAGATCATTGC | ATACTCCTGCTTGCTGATCCAC |
| Claudin-1 | TCTATGACCCTATGACCCCAGT | TCTGGGAAATGATGGCACTAGC |
| Claudin-3 | CGAGAAGAAGTACACGGCCAC | GTCTGTCCCTTAGACGTAGTCC |
| Occludin | CATTAACTTCGCCTGTGGATGAC | TCTCTTTGACCTTCCTGCTCTTC |
| ZO-1 | AGTACCAGAAATACCTGACGGTG | CTTGGCTGACACTAGAAGTAGCA |
| MYD88 | TCGAAAAGAGGTTGGCTAGAAGG | CTTGCTCTGCAGGTAATCATCAG |
| IRF-7 | CCCATCTTCGACTTCAGAGTCTT | CGAAGCCCAGGTAGATGGTATAG |
| NFκB | AGCTTCAGAATGGCAGAAGATGA | CAGTGCCATCTGTGGTTGAAATA |
| TRAF6 | TTGCTCTTATGGATTGTCCCCAA | GACAGTTCTGGTCATGGATCTCT |
| TAK1 | GAGATCAAGAGGGTGATGCAGAT | CGAGTGATAAGCACATTAGCAGC |
| JUK | GCCACAAAATCCTCTTTCCAGG | AGGACATCAGGGAAGAGTTTCTC |
| MEKK | TCACGAAGGAATCAAGAGAGCAA | AAAATAAGCAGCCAACGAGTTCC |
| TBK1 | ACAGATTTTGGTGCAGCTAGAGA | TACCCCAATGCTCCAAAGATCAA |
| IRF-3 | CAAAGAAGGGTTGCGTTTAGCA | ACTCCAGATATTGCACCAGAAGG |
| Arp3 | GCTGCATGAAAATTCAGTTCGTC | GCCACAGAGAAGATTCTTAGCCT |
| RhoA | TCCGGAAGAAACTGGTGATTGTT | TCAGGCGATCATAATCTTCCACA |
| IRSP53 | CGACTCCTACTCCAACACACTC | CAGAGTCTTGTTCTCGGTGGTG |
| cdc42 | CAGAAGCCTATCACTCCAGAGAC | GCAGCCAATATTGCTTCGTCAAA |
| Par3 | CTAATTGGCCTCTCCACTTCTGT | TCCCATCCTCATCCTTCCTGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yu, L.; Zhai, Q.; Liu, B.; Zhao, J.; Chen, W.; Tian, F. Ganoderma lucidum Ethanol Extraction Promotes Dextran Sulphate Sodium Induced Colitis Recovery and Modulation in Microbiota. Foods 2022, 11, 4023. https://doi.org/10.3390/foods11244023
Li M, Yu L, Zhai Q, Liu B, Zhao J, Chen W, Tian F. Ganoderma lucidum Ethanol Extraction Promotes Dextran Sulphate Sodium Induced Colitis Recovery and Modulation in Microbiota. Foods. 2022; 11(24):4023. https://doi.org/10.3390/foods11244023
Chicago/Turabian StyleLi, Miaoyu, Leilei Yu, Qixiao Zhai, Bingshu Liu, Jianxin Zhao, Wei Chen, and Fengwei Tian. 2022. "Ganoderma lucidum Ethanol Extraction Promotes Dextran Sulphate Sodium Induced Colitis Recovery and Modulation in Microbiota" Foods 11, no. 24: 4023. https://doi.org/10.3390/foods11244023