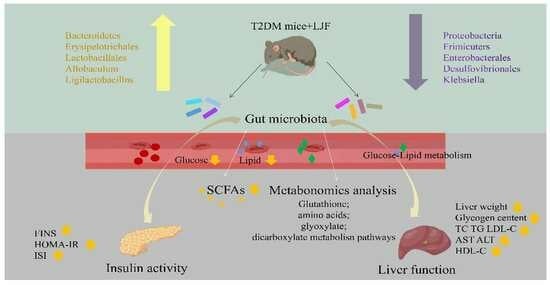
Fucoidan from Laminaria japonica Ameliorates Type 2 Diabetes Mellitus in Association with Modulation of Gut Microbiota and Metabolites in Streptozocin-Treated Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Mice Experiment
2.2.1. Ethics Statement
2.2.2. Experimental Protocol
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Measurement of Biochemical Indexes in Serum
2.5. Histology Analysis
2.6. Analysis of Short Chain Fatty Acids (SCFAs)
2.7. Sequencing Analysis of the Gut Microbiota Composition
2.8. Untargeted and Targeted Metabonomics Analysis
2.9. Statistical Analysis
3. Results
3.1. LJF Regulated Weight Gain and Glucose Tolerance in Mice
3.2. LJF Attenuated Lipid Metabolism Abnormality in T2DM Mice
3.3. LJF Improved Glycogen Content and Oxidative Stress in the Liver
3.4. LJF Regulated the Imbalance between Insulin Resistance and Insulin Sensitivity
3.5. LJF Increased the Production of SCFAs in T2DM Mice
3.6. LJF Modulated Dysbiosis of the Gut Microbiota in T2DM Mice
3.7. LJF Modulated the Microbiota Metabolites Profile in T2DM Mice
3.8. Correlation Analysis between Bacterial Genera and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| LJF | Laminaria japonica fucoidan |
| HFD | High fat diet |
| STZ | Streptozocin |
| T2DM | Type 2 diabetes mellitus |
| FBG | Fasting blood glucose |
| OGTT | Oral glucose tolerance test |
| TC | Total cholesterol |
| TG | Triglyceride |
| HDL-C | High density lipoprotein cholesterol |
| LDL-C | Low density lipoprotein cholesterol |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| FINS | Fasting serum insulin |
| HOMA | Homeostasis model assessment |
| IR | Insulin resistance |
| ISI | Insulin sensitivity index |
| FFA | Free fatty acids |
| GLP-1 | Glucagon-like peptide 1 |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| T-AOC | Total antioxidant capacity |
| CAT | Catalase |
| SCFAS | Short chain fatty acids |
| TCA | Tricarboxylic acid |
| TDCA | Taurodeoxycholic acid |
| TUDCA | Tauroursodeoxycholic acid |
| TCDCA | Taurochenodeoxycholic acid |
| T-MCA | Tauro-muricholic acid |
| LCA | Lymphocyte common antigen |
| HCA | Hydroxycitric acid |
References
- LeRoith, D.; Smith, D.O. Monitoring glycemic control: The cornerstone of diabetes care. Clin. Ther. 2005, 27, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Utzschneher, K.M.; Hull, R.L.; Tong, J.; Wallace, T.M.; Kodama, K.; Shofer, J.B. American Diabetes Association GENNID Study Group, Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006, 29, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Watanabe, R.M. Pharmacogenetics of Anti-Diabetes Drugs. Pharmaceuticals 2010, 3, 2610–2646. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Franz, M.J. Diabetes Nutrition Therapy: Effectiveness, Macronutrients, Eating Patterns and Weight Management. Am. J. Med. Sci. 2016, 351, 374–379. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Evert, A.B. Evidence-based diabetes nutrition therapy recommendations are effective: The key is individualization. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 65–72. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahrami, G.; Daglia, M.; Nabavi, S.M. Plant-Derived Supplementary Carbohydrates, Polysaccharides and Oligosaccharides in Management of Diabetes Mellitus: A Comprehensive Review. Food Rev. Int. 2019, 35, 563–586. [Google Scholar] [CrossRef]
- Shirosaki, M.; Koyama, T. Laminaria japonica as a Food for the Prevention of Obesity and Diabetes. Adv. Food Nutr. Res. 2011, 64, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Ryu, H.-K.; Han, J.S. Effects of Laminaria Japonica Extract Supplement on Blood Glucose, Serum Lipids and Antioxidant Systems in Type ll Diabetic Patients. J. Korean Soc. Food Sci. Nutr. 2007, 36, 1391–1398. [Google Scholar] [CrossRef]
- Jia, X.B.; Yang, J.; Wang, Z.; Liu, R.C.; Xie, R.J. Polysaccharides from Laminaria japonica show hypoglycemic and hypolipidemic activities in mice with experimentally induced diabetes. Exp. Biol. Med. 2014, 239, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhang, L.P.; Qin, V.; Wang, Y.F.; Chen, F.S. Physicochemical Properties of the Soluble Dietary Fiber from Laminaria japonica and Its Role in the Regulation of Type 2 Diabetes Mice. Nutrients 2022, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.J.; Kuo, C.M.; Chou, F.L.; Mi, C.H.; Cheng, C.W. Fucoidan from Laminaria japonica exerts antitumor effects on angiogenesis and micrometastasis in triple-negative breast cancer cells. Int. J. Biol. Macromol. 2020, 149, 600–608. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Cai, J.; Ji, J.; Zhang, W. Polysaccharides from Armillariella tabescens mycelia ameliorate insulin resistance in type 2 diabetic mice. Food Funct. 2020, 11, 9675–9685. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Ai, C.; Tian, W.; Wen, C.; Song, S.; Zhu, B. An acidic polysaccharide from Patinopecten yessoensis skirt prevents obesity and improves gut microbiota and metabolism of mice induced by high-fat diet. Food Res. Int. 2022, 154, 110980. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014, 37 (Suppl. 1), S120–S143. [Google Scholar] [CrossRef]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, L.; Wang, B.; Zhang, Z.; Liu, H.; Zhang, Y.; Liu, J. Synergistic Hypoglycemic Effects of Pumpkin Polysacc harides and Puerarin on Type II Diabetes Mellitus Mice. Molecules 2019, 24, 955. [Google Scholar] [CrossRef]
- Hassan, A.; Tajuddin, N.; Shaikh, A. Retrospective Case Series of Patients with Diabetes or Prediabetes Who Were Switched from Omega-3-Acid Ethyl Esters to Icosapent Ethyl. Cardiol. Ther. 2015, 4, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Madsbad, S.; Bojsen-Moller, K.N.; Svane, M.S.; Jorgensen, N.B. Martinussen, Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg. Obes. Relat. Dis. 2018, 14, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Tonelli, J.; Kishore, P.; Stein, D.; Ragucci, E.; Gitig, A. Contribution of elevated free fatty acid levels to the lack of glucose effectiveness in type 2 diabetes. Diabetes 2003, 52, 2748–2758. [Google Scholar] [CrossRef]
- Choi, E.; Kikuchi, S.; Gao, H.S.; Brodzik, K.; Nassour, I.; Yopp, A. Mitotic regulators and the SHP2-MAPK pathway promote IR endocytosis and feedback regulation of insulin signaling. Nat. Commun. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, X.; You, L. Antihyperglycemic and antihyperlipidemic activities of a polysaccharide from Physalis pubescens L. in streptozotocin (STZ)-induced diabetic mice. Food Funct. 2019, 10, 4868–4876. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Leonard, P. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M. Dietary intervention impact on gut microbial gene richness. Nature 2019, 13500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef]
- Chen, X.Y.; Tan, F.; Yi, R.K.; Mu, J.F.; Zhao, X.; Yang, Z.N. Effects of Lactobacillus on Mice with Diabetes Induced by High-Fat Diet with Streptozotocin (STZ). Appl. Sci. 2018, 8, 1249. [Google Scholar] [CrossRef]
- Dang, F.F.; Jiang, Y.J.; Pan, R.L.; Zhou, Y.H.; Wu, S.; Wang, R.; Zhuang, K.J.; Zhang, W.; Li, T.J.; Man, C.X. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Rahman, U.U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. 2021, 62, 6034–6054. [Google Scholar] [CrossRef]
- Tang, R.; Li, L. Modulation of Short-Chain Fatty Acids as Potential Therapy Method for Type 2 Diabetes Mellitus. Can. J. Infect. Dis. Med. 2021, 2021, 6632266. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Song, L.; Xiao, Y.; Lu, S.; Xu, J.; Li, J.; Ren, Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J. Funct. Foods 2020, 73, 104103. [Google Scholar] [CrossRef]
- Holm, L.J.; Buschard, K. L-serine: A neglected amino acid with a potential therapeutic role in diabetes. APMIS 2019, 127, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A. Branched chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. Available online: https://www.nature.com/articles/srep20594 (accessed on 5 February 2016). [CrossRef] [PubMed]
- Newsholme, P.; Brennan, L.; Rubi, B.; Maechler, P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin. Sci. 2005, 108, 185–194. [Google Scholar] [CrossRef]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. Available online: https://pubmed.ncbi.nlm.nih.gov/27356939 (accessed on 1 June 2017). [CrossRef]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2010, 34, 162–167. [Google Scholar] [CrossRef]
- Proffitt, C.; Bidkhori, G.; Lee, S.; Tebani, A.; Mardinoglu, A.; Uhlen, M.; Moyes, D.L.; Shoaie, S. Genome-scale metabolic modelling of the human gut microbiome reveals changes in the glyoxylate and dicarboxylate metabolism in metabolic disorders. Iscience 2022, 25, 104513. [Google Scholar] [CrossRef]
- Xia, H.; Tang, H.; Wang, F.; Yang, X.; Wang, Z.; Liu, H.; Pan, D.; Yang, C.; Wang, S.; Sun, G. An untargeted metabolomics approach reveals further insights of Lycium barbarum polysaccharides in high fat diet and streptozotocin-induced diabetic rats. Food Res. Int. 2019, 116, 20–29. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Jia, J.; Zhang, P.; Zheng, W.; Guo, X.; Ai, C.; Song, S. Fucoidan from Laminaria japonica Ameliorates Type 2 Diabetes Mellitus in Association with Modulation of Gut Microbiota and Metabolites in Streptozocin-Treated Mice. Foods 2023, 12, 33. https://doi.org/10.3390/foods12010033
Zhang C, Jia J, Zhang P, Zheng W, Guo X, Ai C, Song S. Fucoidan from Laminaria japonica Ameliorates Type 2 Diabetes Mellitus in Association with Modulation of Gut Microbiota and Metabolites in Streptozocin-Treated Mice. Foods. 2023; 12(1):33. https://doi.org/10.3390/foods12010033
Chicago/Turabian StyleZhang, Chenxi, Jinhui Jia, Panpan Zhang, Weiyun Zheng, Xiaoming Guo, Chunqing Ai, and Shuang Song. 2023. "Fucoidan from Laminaria japonica Ameliorates Type 2 Diabetes Mellitus in Association with Modulation of Gut Microbiota and Metabolites in Streptozocin-Treated Mice" Foods 12, no. 1: 33. https://doi.org/10.3390/foods12010033