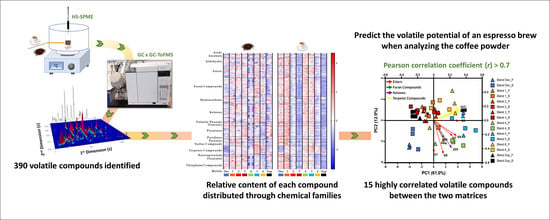
Insights on Single-Dose Espresso Coffee Capsules’ Volatile Profile: From Ground Powder Volatiles to Prediction of Espresso Brew Aroma Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Capsules under Study and Espresso Coffee Preparation
2.2. HS-SPME Experimental Conditions
- Coffee powders: the analysis of the powdered coffees was performed based on a previous study [29]. Briefly, for each HS-SPME assay, 1.2 g of sample was placed into a 12 mL glass vial that was capped with a PTFE septum and a screw cap (Chromacol, Hertfordshire, UK). The vial was placed in a thermostatic bath adjusted to 55.0 ± 0.1 °C, and the SPME fiber DVB/CAR/PDMS was inserted into the headspace for 12 min.
- Espresso coffee brew: the SPME assay used for the espresso coffee brew was defined to simulate the consumer’s perception when drinking an espresso coffee. So, based on a previously reported work [30], each espresso coffee sample (40 mL ± 2) was extracted directly into a thermostatized SPME glass vial (120 mL, 60.0 ± 0.1 °C, for 5 min), which was sealed and kept at 60 °C, at constant stirring (ca. 400 rpm), and the DVB/CAR/PDMS fiber was immediately inserted into the sample headspace for 3 min. The temperature of 60 °C was chosen since it is about the same temperature as the coffee would normally be when consumed by the consumer [17].
2.3. GC × GC-ToFMS Analysis
2.4. Data Processing
3. Results and Discussion
3.1. Volatile Composition of Single-Dose Espresso Coffee-Based Blends
3.2. Multivariate Analysis of Coffee Powders and Brews
3.3. Coffee Powder Discriminant Volatiles That Predict Brews’ Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- References Yeretzian, C.; Opitz, S.; Smrke, S.; Wellinger, M. Coffee volatile and aroma compounds—From the green bean to the cup. In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 726–770. [Google Scholar]
- Lopez-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006, 54, 8560–8566. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef]
- Pereira, G.; Neto, D.; Magalhaes, A.; Vasquez, Z.; Medeiros, A.; Vandenberghe, L.; Soccol, C. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.; Pezza, L.; Pezza, H.; Toci, A. Relationship between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, M.; Shellie, R.; Keast, R. Unravelling the relationship between aroma compounds and consumer acceptance: Coffee as an example. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2380–2420. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Shellie, R.; Tranchida, P.; Casilli, A.; Mondello, L.; Marriott, P. Analysis of roasted coffee bean volatiles by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2004, 1054, 57–65. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzanski, B.; Oniszczuk, T.; Combrzynski, M.; Cwikla, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries Using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef] [PubMed]
- Ongo, E.; Montevecchi, G.; Antonelli, A.; Sberveglieri, V.; Sevilla, F. Metabolomics fingerprint of Philippine coffee by SPME-GC-MS for geographical and varietal classification. Food Res. Int. 2020, 134, 109227. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Reichenbach, S.; Tian, X.; Tao, Q. Targeted and Non-Targeted Approaches for Complex Natural Sample Profiling by GCxGC-qMS. J. Chromatogr. Sci. 2010, 48, 251–261. [Google Scholar] [CrossRef]
- Bodner, M.; Morozova, K.; Kruathongsri, P.; Thakeow, P.; Scampicchio, M. Effect of harvesting altitude, fermentation time and roasting degree on the aroma released by coffee powder monitored by proton transfer reaction mass spectrometry. Eur. Food Res. Technol. 2019, 245, 1499–1506. [Google Scholar] [CrossRef]
- Petisca, C.; Perez-Palacios, T.; Farah, A.; Pinho, O.; Ferreira, I. Furans and other volatile compounds in ground roasted and espresso coffee using headspace solid-phase microextraction: Effect of roasting speed. Food Bioprod. Process. 2013, 91, 233–241. [Google Scholar] [CrossRef]
- Maeztu, L.; Sanz, C.; Andueza, S.; De Pena, M.; Bello, J.; Cid, C. Characterization of espresso coffee aroma by static headspace GC-MS and sensory flavor profile. J. Agric. Food Chem. 2001, 49, 5437–5444. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. Int. J. Food Sci. Nutr. 2015, 66, 505–513. [Google Scholar] [CrossRef]
- Rocha, S.; Maeztu, L.; Barros, A.; Cid, C.; Coimbra, M. Screening and distinction of coffee brews based on headspace solid phase microextraction/gas chromatography/principal component analysis. J. Sci. Food Agric. 2004, 84, 43–51. [Google Scholar] [CrossRef]
- Laukalēja, I.; Krūma, Z. Evaluation of headspace solid-phase microextraction with different fibres for volatile compound determination in specialty coffee brews. Food Sci. 2019, 1, 215–221. [Google Scholar] [CrossRef]
- Giacalone, D.; Degn, T.; Yang, N.; Liu, C.; Fisk, I.; Munchow, M. Common roasting defects in coffee: Aroma composition, sensory characterization and consumer perception. Food Qual. Prefer. 2019, 71, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Welke, J.; Zini, C. Comprehensive Two-Dimensional Gas Chromatography for Analysis of Volatile Compounds in Foods and Beverages. J. Braz. Chem. Soc. 2011, 22, 609–622. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R.; Tranchida, P.; Dugo, P.; Lo Presti, M.; Festa, S.; Fazio, A.; Dugo, G. Reliable characterization of coffee bean aroma profiles by automated headspace solid phase microextraction-gas chromatography-mass spectrometry with the support of a dual-filter mass spectra library. J. Sep. Sci. 2005, 28, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on coffee aroma: II. Roasted coffee. J. Food Compos. Anal. 2007, 20, 297–307. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Hirano, Y.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Characterization of headspace aroma compounds of freshly brewed arabica coffees and studies on a characteristic aroma compound of Ethiopian coffee. J. Food Sci. 2008, 73, C335–C346. [Google Scholar] [CrossRef]
- Sarghini, F.; Fasano, E.; De Vivo, A.; Tricarico, M.C. Influence of Roasting Process in Six Coffee Arabica Cultivars: Analysis of Volatile Components Profiles. Chem. Eng. Trans. 2019, 75, 295–300. [Google Scholar] [CrossRef]
- Lopes, G.; Ferreira, A.; Pinto, M.; Passos, C.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.; Nunes, F.; Coimbra, M. Carbohydrate content, dietary fibre and melanoidins: Composition of espresso from single-dose coffee capsules. Food Res. Int. 2016, 89, 989–996. [Google Scholar] [CrossRef]
- Parenti, A.; Guerrini, L.; Masella, P.; Spinelli, S.; Calamai, L.; Spugnoli, P. Comparison of espresso coffee brewing techniques. J. Food Eng. 2014, 121, 112–117. [Google Scholar] [CrossRef]
- Lolli, V.; Acharjee, A.; Angelino, D.; Tassotti, M.; Del Rio, D.; Mena, P.; Caligiani, A. Chemical Characterization of Capsule-Brewed Espresso Coffee Aroma from the Most Widespread Italian Brands by HS-SPME/GC-MS. Molecules 2020, 25, 1166. [Google Scholar] [CrossRef] [Green Version]
- Petisca, C.; Perez-Palacios, T.; Pinho, O.; Ferreira, I. Optimization and Application of a HS-SPME-GC-MS Methodology for Quantification of Furanic Compounds in Espresso Coffee. Food Anal. Methods 2014, 7, 81–88. [Google Scholar] [CrossRef]
- Rahn, A.; Yeretzian, C. Impact of consumer behavior on furan and furan-derivative exposure during coffee consumption. A comparison between brewing methods and drinking preferences. Food Chem. 2019, 272, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, F.; Tripodi, G.; Merlin, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT-Food Sci. Technol. 2020, 118, 108718. [Google Scholar] [CrossRef]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase microextraction–gas chromatographic-time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Petronilho, S.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Carbohydrates as targeting compounds to produce infusions resembling espresso coffee brews using quality by design approach. Food Chem. 2021, 344, 128613. [Google Scholar] [CrossRef] [PubMed]
- Vandendool, H.; Kratz, P. A generalization of retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flament, I.; Bessière-Thomas, Y. Coffee Flavor Chemistry; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Arctander, S. Perfume and Flavor Chemicals: (Aroma Chemicals); Allured Publishing Corporation: Montclair, NJ, USA, 1969. [Google Scholar]
- Akwama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Analysis of the headspace volatiles of freshly brewed arabica coffee using solid-phase microextraction. J. Food Sci. 2007, 72, C388–C396. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Chin, S.T.; Eyres, G.T.; Marriott, P.J. Identification of potent odourants in wine and brewed coffee using gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 7487–7498. [Google Scholar] [CrossRef]
- Czerny, M.; Grosch, W. Potent odorants of raw Arabica coffee. Their changes during roasting. J. Agric. Food Chem. 2000, 48, 868–872. [Google Scholar] [CrossRef]
- Blank, I.; Sen, A.; Grosch, W. Potent Odorants of the Roasted Powder and Brew of Arabica Coffee. Z. Lebensm.-Unters. Forsch. 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Lindinger, C.; Pollien, P.; de Vos, R.; Tikunov, Y.; Hageman, J.; Lambot, C.; Fumeaux, R.; Voirol-Baliguet, E.; Blank, I. Identification of Ethyl Formate as a Quality Marker of the Fermented Off-note in Coffee by a Nontargeted Chemometric Approach. J. Agric. Food Chem. 2009, 57, 9972–9978. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on the volatile fraction of coffee beans: I. Green coffee. J. Food Compos. Anal. 2007, 20, 289–296. [Google Scholar] [CrossRef]
- Cantergiani, E.; Brevard, H.; Krebs, Y.; Feria-Morales, A.; Amadò, R.; Yeretzian, C. Characterisation of the aroma of green Mexican coffee and identification of mouldy/earthy defect. Eur. Food Res. Technol. 2001, 212, 648–657. [Google Scholar] [CrossRef]
- Cordero, C.; Bicchi, C.; Rubiolo, P. Group-type and fingerprint analysis of roasted food matrices (coffee and hazelnut samples) by comprehensive two-dimensional gas chromatography. J. Agric. Food Chem. 2008, 56, 7655–7666. [Google Scholar] [CrossRef] [PubMed]
- Semmelroch, P.; Grosch, W. Analysis of roasted coffee powders and brews by gas chromatography-olfactometry of headspace samples. LWT Food Sci. Technol. 1995, 28, 310–313. [Google Scholar] [CrossRef]
- Hofmann, T.; Schieberle, P. Chemical Interactions between Odor-Active Thiols and Melanoidins Involved in the Aroma Staling of Coffee Beverages. J. Agric. Food Chem. 2002, 50, 319–326. [Google Scholar] [CrossRef]
- Holscher, W.; Steinhart, H. Investigation of roasted coffee freshness with an improved headspace technique. Z. Lebensm.-Unters. Forsch. 1992, 195, 33–38. [Google Scholar] [CrossRef]
- Sanz, C.; Ansorena, D.; Bello, J.; Cid, C. Optimizing headspace temperature and time sampling for identification of volatile compounds in ground roasted Arabica coffee. J. Agric. Food Chem. 2001, 49, 1364–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebesny, E.; Budryn, G.; Kula, J.; Majda, T. The effect of roasting method on headspace composition of robusta coffee bean aroma. Eur. Food Res. Technol. 2007, 225, 9–19. [Google Scholar] [CrossRef]
- Piccino, S.; Boulanger, R.; Descroix, F.; Sing, A. Aromatic composition and potent odorants of the “specialty coffee” brew “Bourbon Pointu” correlated to its three trade classifications. Food Res. Int. 2014, 61, 264–271. [Google Scholar] [CrossRef]
- Holscher, W.; Steinhart, H. Formation Pathways for Primary Roasted Coffee Aroma Compounds. In Thermally Generated Flavors; Parliament, T.H., Morello, M.J., McGorrin, R.J., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 543, pp. 206–217. [Google Scholar]
- Lopez-Darias, J.; Anderson, J.; Pino, V.; Afonso, A. Developing qualitative extraction profiles of coffee aromas utilizing polymeric ionic liquid sorbent coatings in headspace solid-phase microextraction gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H.; Sagara, Y. Analysis of Freshly Brewed Espresso Using a Retronasal Aroma Simulator and Influence of Milk Addition. Food Sci. Technol. Res. 2009, 15, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Sanz, C.; Maeztu, L.; Zapelena, M.; Bello, J.; Cid, C. Profiles of volatile compounds and sensory analysis of three blends of coffee: Influence of different proportions of Arabica and Robusta and influence of roasting coffee with sugar. J. Sci. Food Agric. 2002, 82, 840–847. [Google Scholar] [CrossRef]
- Zapata, J.; Londono, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an industrial recovery concentrate of coffee flavour. Cyta J. Food 2018, 16, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.; Neto, E.; Soccol, V.; Medeiros, A.; Woiciechowski, A.; Soccol, C. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- Evangelista, S.; Silva, C.; Miguel, M.; Cordeiro, C.; Pinheiro, A.; Duarte, W.; Schwan, R. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, S.; Miguel, M.; Cordeiro, C.; Silva, C.; Pinheiro, A.; Schwan, R. Inoculation of starter cultures in a semi-dry coffee (Coffea Arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S. Coffee flavour modification through controlled fermentations of green coffee beans by Saccharomyces cerevisiae and Pichia kluyveri: Part, I. Effects from individual yeasts. Food Res. Int. 2020, 136, 109588. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ohtani, N.; Iwatsuki, K.; Sotoyama, K.; Wada, A.; Tokuno, K.; Iwabuchi, H.; Tanaka, K. Analysis of volatile compounds released during the grinding of roasted coffee beans using solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Flament, I. Coffee Flavor Chemistry; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Ascrizzi, R.; Flamini, G. Wild Harenna coffee: Flavour profiling from the bean to the cup. Eur. Food Res. Technol. 2020, 246, 643–660. [Google Scholar] [CrossRef]
- Holscher, W.; Steinhart, H. Aroma compounds in green coffee. In Developments in Food Science; George, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 785–803. [Google Scholar]
- Cui, D.; Liu, Y.; Chen, Y.; Feng, X.; Lu, Y.; Yu, B. Application of SPME-GC-TOFMS, E-nose, and sensory evaluation to investigate the flavor characteristics of Chinese Yunnan coffee at three different conditions (beans, ground powder, and brewed coffee). Flavour Fragr. J. 2020, 35, 541–560. [Google Scholar] [CrossRef]
- Lee, L.; Tay, G.; Cheong, M.; Curran, P.; Yu, B.; Liu, S. Modulation of the volatile and non-volatile profiles of coffee fermented with Yarrowia lipolytica: II. Roasted coffee. LWT Food Sci. Technol. 2017, 80, 32–42. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Identification of odor-active 3-mercapto-3-methylbutyl acetate in volatile fraction of roasted coffee brew isolated by steam distillation under reduced pressure. J. Agric. Food Chem. 2003, 51, 3079–3082. [Google Scholar] [CrossRef]
- Farag, M.; Otify, A.; El-Sayed, A.; Michel, C.; ElShebiney, S.; Ehrlich, A.; Wessjohann, L. Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics. Molecules 2019, 24, 3377. [Google Scholar] [CrossRef] [Green Version]
- Toci, A.; Farah, A. Volatile fingerprint of Brazilian defective coffee seeds: Corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef]
- Pickard, S.; Becker, I.; Merz, K.; Richling, E. Determination of the Alkylpyrazine Composition of Coffee Using Stable Isotope Dilution-Gas Chromatography-Mass Spectrometry (SIDA-GC-MS). J. Agric. Food Chem. 2013, 61, 6274–6281. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.R.; Ferreira, A.; Pinto, M.; Passos, C.; Coelho, E.; Rocha, S.M.; Coimbra, M.A. Single-dose espresso coffee capsules: A complete data set characterization of body, color and aroma. In Proceedings of the 25th International Conference on Coffee Science, Association Scientifique International du Café (ASIC), Armenia, Colombia, 8–13 September 2014. [Google Scholar]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhumiratana, N.; Adhikari, K.; Chambers, E. Evolution of sensory aroma attributes from coffee beans to brewed coffee. LWT Food Sci. Technol. 2011, 44, 2185–2192. [Google Scholar] [CrossRef] [Green Version]





| Compound | CAS Number | Reported a | Aroma Descriptor b | References c |
|---|---|---|---|---|
| Acids | ||||
| Aliphatics | ||||
| Acetic acid | 64-19-7 | [33] | Pungent, sour, acidic, vinegar | [20,34,35,36] |
| Propanoic acid | 79-09-4 | [33] | Pungent, rancid, sour milk, cheese, butter-like | [34,36] |
| Butanoic acid | 107-92-6 | [33] | Sour, rancid, butter-like, sweaty, rubbish | [34,36,37,38] |
| Isovaleric acid | 503-74-2 | [33] | Acidic, cheesy, herbaceous, sweaty, rancid | [34,35,36,37,39] |
| Alcohols | ||||
| Aliphatics | ||||
| 2-Methyl-1-propanol | 78-83-1 | [33] | Wine-like | [36] |
| 3-Buten-1-ol | 627-27-0 | - | ||
| 3-Methyl-3-buten-1-ol | 763-32-6 | [33] | - | |
| 2-Methyl-1-butanol | 137-32-6 | [40] | Cooked, roasted with fruity or alcoholic undernotes | [36] |
| 1-Pentanol | 71-41-0 | [33] | Green, chemical, fusel oil-like sweet | [34,36,41] |
| 3-Methyl-2-buten-1-ol | 556-82-1 | [33] | Fresh, herbaceous-fruity-green, lavender-like, phenolic, metallic | [34,36] |
| 2-Hexanol | 626-93-7 | [33] | - | |
| 2-Heptanol | 543-49-7 | [33] | Fresh, lemon-like, grassy-herbaceous, sweet-floral undertone | [34,36] |
| 1-Octen-3-ol | 3391-86-4 | [33] | Mushroom, herbaceous, savory, brothy, meaty | [37,41,42] |
| 2-Ethyl-1-hexanol | 104-76-7 | [13] | Sweet, slightly floral rose-like | [36] |
| 1-Octanol | 111-87-5 | [43] | Fresh, orange-rose, sweet | [36] |
| Aromatics | ||||
| 2-Phenylethanol | 60-12-8 | [33] | Rose-honey-like, floral | [34,36,41] |
| Aldehydes | ||||
| Aliphatics | ||||
| Acetaldehyde | 75-07-0 | [33] | Pungent, ethereal, fruity, coffee, wine, acrid/egg | [34,36,41,44,45] |
| 2-Methylpropanal | 78-84-2 | [33] | Pungent, sour, fruity, malty, buttery-oily | [34,36,46] |
| 2-Butenal | 4170-30-3 | [47,48] | - | |
| 3-Methylbutanal | 590-86-3 | [33] | Pungent, acrid, fruity, apple-like, almond, malty, sweaty | [34,36,39,44,46] |
| 2-Methyl-2-butenal | 1115-11-3 | [33] | - | |
| 2-Pentenal | 764-39-6 | Pungent, green, apple, orange, tomato | [36] | |
| Hexanal | 66-25-1 | [33] | Fatty, green, grassy, fruity, rancid butter-like, nutty | [34,36,38,41,46] |
| 4-Methyl-3-pentenal | 5362-50-5 | - | ||
| 2-Methyl-2-hexenal | 28467-88-1 | - | ||
| 2,4-Hexadienal | 80466-34-8 | [33] | Fresh, green, floral, citrus | [36] |
| 4-Methylhexanal | 41065-97-8 | - | ||
| Heptanal | 111-71-7 | [33] | Oily-fatty, rancid, pungent, fermented-fruit-like | [34,36] |
| 2-Heptenal | 2463-63-0 | [43] | Pungent, green, fatty | [36] |
| Octanal | 124-13-0 | [33] | Fatty, citrus, orange-like, honey | [2,36] |
| 2,4-Heptadienal | 5910-85-0 | [49] | Fatty, green | [36] |
| 2-Octenal | 2363-89-5 | [37] | Green-leafy, orange, honey-like, cognac-like | [36,37] |
| Nonanal | 124-19-6 | [19,43] | Fatty, orange and rose note, soap-like, metallic | [36,50] |
| 2-Nonenal | 2463-53-8 | [33] | Fatty, orris-like, waxy, dried orange peel-like, cardboard-like | [36,38] |
| Decanal | 112-31-2 | [19,49,51] | Sweet, waxy, floral, citrus, fatty | [36] |
| Undecanal | 112-44-7 | [51] | Sweet, fatty, orange and rose undertone | [36] |
| Dodecanal | 112-54-9 | [49,51] | Fatty, violet-like | [36] |
| Aromatics | ||||
| Benzaldehyde | 100-52-7 | [33] | Sweet, bitter almond-like, bitter | [34,36,41] |
| Benzeneacetaldehyde | 122-78-1 | [33] | Pungent-green, hyacinth-like, floral, sweet-fruity, honey-like | [34,36,39,50,52] |
| 2-Hydroxybenzaldehyde | 90-02-8 | [33] | Pungent, herbaceous, spicy-floral, bitter, almond-like | [34,36] |
| 2-Methylbenzaldehyde | 529-20-4 | [33] | Sweet, beany, fresh pea | [34,37] |
| 2-Phenyl-2-butenal | 4411-89-6 | [33] | Musty, floral, cocoa | [4] |
| Esters | ||||
| Aliphatics | ||||
| Methyl acetate | 79-20-9 | [33] | Sweet, ethereal, fruity | [34,36] |
| Methyl propenoate | 96-33-3 | - | ||
| Methyl propanoate | 554-12-1 | [33] | Ethereal-rum-like, sweet, fruity | [34,36] |
| Methyl glycolate | 96-35-5 | [47] | - | |
| Methyl 2-methylpropenoate | 80-62-6 | Acrid, fruity | [36] | |
| Methyl butanoate | 623-42-7 | [33] | Sweet-ethereal, fruity, apple peel, peach-like | [34,36] |
| Methyl 2-butenoate | 18707-60-3 | [48] | - | |
| Methyl 2-methylbutanoate | 868-57-5 | Sweet, fruity, apple-like | [36] | |
| Methyl 3-methylbutanoate | 556-24-1 | [33] | Ethereal, fruity, apple-like, herbaceous | [34,36] |
| 3-Methylbutyl formate | 110-45-2 | [37] | Plum, fruity, black currant-like | [36,37] |
| Butyl acetate | 123-86-4 | [33] | Pear-like, ethereal, fruity, ripe/over-ripe fruits-like | [34,36] |
| Methyl pentanoate | 624-24-8 | Ethereal, green-fruity, apple-like, pineapple- like | [34,36] | |
| Methyl 3-methyl-2-butenoate | 924-50-5 | [53] | roasted | [54] |
| Ethyl 3-methylbutanoate | 108-64-5 | [33] | Fruity, apple-like | [36,38,41] |
| Isoamyl acetate | 123-92-2 | [33] | Fruity, banana, sweet, apple-like | [34,36] |
| Methyl 3-methylpentanoate | 2177-78-8 | - | ||
| 3-Methyl-3-butenyl acetate | 5205-07-2 | Fruity | [36] | |
| Methyl 4-methylpentanoate | 2412-80-8 | Sweet, pineapple-like | [36] | |
| Pentyl acetate | 628-63-7 | - | ||
| Methyl hexanoate | 106-70-7 | [33] | Pineapple-like, apricot-like, sweet, ethereal | [34,36] |
| Ethyl tiglate | 5837-78-5 | Fruity, caramel | [34,36] | |
| 3-Methyl-2-butenyl acetate | 1191-16-8 | [33] | Fresh, fruity, banana-like, bergamot-like | [34] |
| Hexyl formate | 629-33-4 | Fruity, apple-like, unripe-plum | [36] | |
| Ethylidene acetate | 542-10-9 | - | ||
| Methyl 4-Methyl-2-oxopentanoate | 3682-43-7 | - | ||
| Butyl 2-methyl-2-propenoate | 97-88-1 | - | ||
| Hexyl acetate | 142-92-7 | [55] | Fruity, apple, cherry, pear, floral | [36] |
| Isobutyl 3-methyl-2-butenoate | 30434-54-9 | - | ||
| Methyl octanoate | 111-11-5 | Winy, fruity, orange-like | [36] | |
| 3-Methylbutyl 3-Methyl-2-butenoate | 56922-73-7 | - | ||
| Methyl decanoate | 110-42-9 | - | ||
| Methyl dodecanoate | 111-82-0 | Fatty, floral, wine-like | [36] | |
| Aromatics | ||||
| Phenyl acetate | 122-79-2 | [56,57] | Floral, rosy, dark chocolate-like | [4] |
| Benzyl formate | 104-57-4 | [33] | Fruity, green, herbaceous, earthy, floral | [34,36] |
| Methyl benzoate | 93-58-3 | [33] | Fruity, cananga-like | [36] |
| Methyl phenylacetate | 101-41-7 | [33] | Honey, musky, jasmine, floral | [34,36] |
| 2-Phenylethyl formate | 104-62-1 | [33] | Green, herbaceous, rosy, hyacinth, chrysanthemum, watercress-foliage | [34,36] |
| Methyl 2-hydroxybenzoate | 119-36-8 | [33] | Sweet, rooty-fruity, minty, spicy, wintergreen-like | [34,36] |
| 2-Phenylethyl acetate | 103-45-7 | [58] | Floral, rose, honey-like | [36] |
| Ethyl 2-hydroxybenzoate | 118-61-6 | [48] | Wintergreen | [36] |
| Furan compounds | ||||
| Furan | 110-00-9 | [33] | Spicy-smoky, cinnamon-like | [34] |
| 2-Methylfuran | 534-22-5 | [33] | Ethereal, sickly | [20] |
| Tetrahydrofuran | 109-99-9 | [33] | Sweet-gassy, bread-like | [34] |
| 2,5-Dimethylfuran | 625-86-5 | [33] | coffee | [20] |
| 2,4-Dimethylfuran | 3710-43-8 | [48] | - | |
| 2-Propylfuran | 4229-91-8 | [33] | - | |
| 2-Ethyl-5-methylfuran | 1703-52-2 | [33] | - | |
| 2-Ethyl-5-methyltetrahydrofuran | 931-39-5 | - | ||
| 2-Furancarbonitrile | 617-90-3 | - | ||
| Dihydro-2-methyl-3-furanone | 3188-00-9 | [33] | Bread-like, buttery, nutty | [20,36] |
| 2,3,5-Trimethylfuran | 10504-04-8 | [33] | - | |
| 3-Furaldehyde | 498-60-2 | [33] | - | |
| 2-Vinyl-5-methylfuran | 10504-13-9 | [33] | Coffee | [20] |
| 2-(Methoxymethyl)furan | 13679-46-4 | [33] | Burnt, herbal, potato-like | [2,37] |
| 2,3,4-Trimethylfuran | 10599-57-2 | [33] | - | |
| Furfural | 98-01-1 | [33] | Sweet, bread-like, caramel-like, cinnamon-almond-like, bitter | [20,34] |
| 2-(2-Propenyl)furan | 75135-41-0 | [33] | - | |
| 5-Methyl-2(3H)-furanone | 591-12-8 | [37,59] | Sweet, herbaceous, tobacco-like, coffee, earthy, raw potato skin | [20,36,37] |
| 2-Furanmethanol | 98-00-0 | [33] | Slightly caramel-like, warm, oily, burnt, bitter | [34,36,37,41] |
| 2,5-Diethyltetrahydrofuran | 41239-48-9 | Sweet, herbaceous, caramel-like | [36] | |
| 2-Butylfuran | 4466-24-4 | [33] | - | |
| Furfuryl formate | 13493-97-5 | [33] | Floral | [20] |
| 2-Acetylfuran | 1192-62-7 | [33] | Balsamic-sweet, tobacco-like, floral, balsamic-cinnamic, spicy, roasty | [2,20,34,36] |
| γ-Butyrolactone | 96-48-0 | [33] | Sweet, slightly buttery | [20,34,36] |
| 2,3,4,5-Tetramethylfuran | 10599-58-3 | [33] | - | |
| 3-(1,1-Dimethylethyl)-2,3-dihydrofuran | 34314-82-4 | - | ||
| 1-(2-Furyl)-2-propanone | 6975-60-6 | [33] | Sweet, fruity-caramel-like, spicy, radish | [34,36] |
| 2-Methyl-5-propenylfuran | 5555-95-3 | [33] | Candy, fruity, sweet | [37] |
| Dihydro- 5-methyl-2(3H)-furanone | 108-29-2 | [33] | Sweet, hay-like, tobacco-like, herbaceous | [34,36] |
| 5-Methylfurfural | 620-02-0 | [33] | Sweet, spicy, caramel | [20,34,36,41] |
| 2-Acetyl-5-methylfuran | 1193-79-9 | [33] | Nutty | [36] |
| Methyl 2-furoate | 611-13-2 | [33] | Berry-like, fruity, winey, mushrooms-like, fungus-like, tobacco-like | [34,36] |
| 2-Pentylfuran | 3777-69-3 | [33] | Fruity, green bean, metallic, vegetable | [36] |
| Benzofuran | 271-89-6 | [33] | - | |
| Furfuryl acetate | 623-17-6 | [33] | Ethereal-floral, herbal-spicy, fruity, banana, nutty | [20,34,36] |
| 2,5-Dihydro-3,5-dimethyl-2-furanone | 5584-69-0 | [19,21] | - | |
| 1-(2-Furanyl)-1-propanone | 3194-15-8 | [33] | - | |
| 2,2’-Bifuran | 5905-00-0 | [33] | - | |
| 3,4-Dimethyl-2,5-furandione | 766-39-2 | [33] | - | |
| 1-(5-Methyl-2-furyl)-2-propanone | 13678-74-5 | [33] | - | |
| 1-(2-Furanyl)-2-butanone | 4208-63-3 | [33] | - | |
| 5-Ethyl-2-furaldehyde | 23074-10-4 | [33] | - | |
| 1-(2-Furanyl)-3-butanone | 699-17-2 | [33] | - | |
| 2,2′-Methylenebisfuran | 1197-40-6 | [33] | - | |
| 3-Acetyl-2,5-dimethylfuran | 10599-70-9 | - | ||
| Furfuryl propanoate | 623-19-8 | [33] | Spicy, floral, fruity | [36,60] |
| 1-(2-Furanyl)-1-butanone | 4208-57-5 | [33] | - | |
| 2-Methylbenzofuran | 4265-25-2 | [33] | - | |
| 2-Methyl-5-propionylfuran | 10599-69-6 | [33] | - | |
| 1-(5-Methyl-2-furanyl)-2-butanone | 13678-70-1 | [33] | - | |
| 2-Heptylfuran | 3777-71-7 | [33] | Roasted, nutty | [36] |
| Furfuryl butanoate | 623-21-2 | [33] | - | |
| 2-(2-Furanylmethyl)-5-methylfuran | 13678-51-8 | [33] | - | |
| n-Furfuryl pyrrole | 1438-94-4 | [33] | Vegetable, green, earthy, horseradish, mushroom-like | [36,60] |
| 2-Methyl-3(2-furyl)acrolein | 874-66-8 | - | ||
| 4-(2-Furanyl)-3-buten-2-one | 623-15-4 | [33] | Spicy-woody, sweet, cinnamon-like, balsamic, vanilla, woody | [20,36] |
| 4,7-Dimethylbenzofuran | 28715-26-6 | - | ||
| 2-Furyl pyrazine | 32736-95-1 | [33] | - | |
| 2,2′-Methylenebis(5-methylfuran) | 13679-43-1 | [33] | - | |
| 1-(5-Methylfurfuryl)pyrrole | 13678-52-9 | [33] | Mushroom-like, green, pharmaceutical, roasty | [2,60] |
| Difurfuryl ether | 4437-22-3 | [33] | - | |
| 1-Furfuryl-2-formylpyrrole | 13788-32-4 | [33] | green, minty | [20] |
| 2-Acetyl-1-furfurylpyrrole | 13678-73-4 | [33] | - | |
| Hydrocarbons | ||||
| Aliphatics | ||||
| Nonane | 111-84-2 | [33] | - | |
| 1,3-Nonadiene | - | - | ||
| Decane | 124-18-5 | [33] | - | |
| Undecane | 1120-21-4 | [33] | - | |
| 1-Dodecene | 112-41-4 | - | ||
| Dodecane | 112-40-3 | [33] | - | |
| Tridecane | 629-50-5 | [33] | - | |
| Tetradecane | 629-59-4 | [33] | - | |
| Pentadecane | 629-62-9 | [33] | - | |
| Hexadecane | 544-76-3 | [33] | - | |
| Aromatics | ||||
| Methylbenzene | 108-88-3 | [33] | Sweet-gassy | [34] |
| Ethylbenzene | 100-41-4 | [33] | Sweet-gassy | [34] |
| 1,3-Dimethylbenzene | 108-38-3 | [33] | - | |
| Phenylethylene | 100-42-5 | [33] | Sweet-gassy, balsamic, floral | [34,36] |
| 1-Methylethylbenzene | 98-82-8 | - | ||
| 1-Ethyl-4-methylbenzene | 622-96-8 | [33] | - | |
| 1,2,3-Trimethylbenzene | 526-73-8 | - | ||
| 1-Methyl-3-propylbenzene | 1074-43-7 | - | ||
| Butylbenzene | 104-51-8 | - | ||
| 4-Ethyl-1,2-dimethylbenzene | 934-80-5 | - | ||
| 1-Ethyl-2,3-dimethylbenzene | 933-98-2 | - | ||
| 1,2,4,5-Tetramethylbenzene | 95-93-2 | [33] | - | |
| 1,2,3,4-Tetramethylbenzene | 488-23-3 | [61] | - | |
| Pentylbenzene | 538-68-1 | - | ||
| 1-Butylheptylbenzene | 4537-15-9 | - | ||
| Ketones | ||||
| Aliphatics | ||||
| 2-Propanone | 67-64-1 | [33] | Ethereal, lemon | [34,41] |
| 2-Butanone | 78-93-3 | [33] | Ethereal, sweet apricot-like | [34,36] |
| 2,3-Butanedione (Diacetyl) | 431-03-8 | [33] | Buttery | [34,35,36,44,46] |
| 2-Pentanone | 107-87-9 | [33] | Ethereal-fruity, wine | [34,36] |
| 2,3-Pentanedione | 600-14-6 | [33] | Buttery, oily, sweet, caramel-like, milky | [2,20,34,35,36,37,44] |
| 1-Hydroxy-2-propanone | 116-09-6 | [33] | Sweet-caramel-like, mushroom, earthy, nutty | [20,34,37] |
| 4-Methyl-2-pentanone | 108-10-1 | [33] | Ethereal-fruity, spicy | [34,36] |
| 3-Penten-2-one | 625-33-2 | [33] | Fruity | [36] |
| 3-Hydroxy-2-butanone (Acetoin) | 513-86-0 | [33] | Creamy-fatty-buttery, woody, yogurt | [20,34,36,41] |
| 2-Methyl-1-penten-3-one | 25044-01-3 | - | ||
| 2,4-Pentanedione | 123-54-6 | [33] | Ethereal-minty, metallic | [34] |
| 3-Hexanone | 589-38-8 | [33] | Ethereal, grape, wine-like | [37] |
| 2,4-Dimethyl-3-pentanone | 565-80-0 | [33] | - | |
| 2-Hexanone | 591-78-6 | [33] | - | |
| 3,4-Hexanedione | 4437-51-8 | [33] | Buttery, toasty, burnt, nutty, caramel-eggy | [34,36] |
| 1-Hydroxy-2-butanone | 5077-67-8 | [33] | Toasted | [20] |
| 3-Hydroxy-2-pentanone | 3142-66-3 | [33] | Earthy, aged | [37] |
| 2-Hydroxy-3-pentanone | 5704-20-1 | [33] | - | |
| 3-Hexen-2-one | 763-93-9 | - | ||
| 3-Hexene-2,5-dione | 4436-75-3 | - | ||
| 4-Methyl-2-hexanone | 105-42-0 | - | ||
| 4-Heptanone | 123-19-3 | [33] | Ethereal-fruity, pineapple-like, strawberry-like | [34] |
| 1-(Acetyloxy)-2-propanone | 592-20-1 | [33] | Fruity-buttery, sour | [34] |
| 3-Heptanone | 106-35-4 | [33] | Green, fatty, fruity, sweet, ethereal | [34,36] |
| 2-Heptanone | 110-43-0 | [33] | Fruity, spicy, cinnamon, banana, spicy | [36] |
| 3-Hepten-2-one | 1119-44-4 | [37] | Green-grassy | [36] |
| 2,5-Hexanedione | 110-13-4 | [33] | Sweet-ethereal | [34] |
| 6-Methyl-3-heptanone | 624-42-0 | - | ||
| 6-Methyl-2-heptanone | 928-68-7 | - | ||
| 5-Methyl-2-heptanone | 18217-12-4 | - | ||
| 1-Octen-3-one | 4312-99-6 | [33] | Mushroom | [36,38,45,62] |
| 2,3-Octanedione | 585-25-1 | [33] | Warmed-over | [36] |
| 6-Methyl-5-hepten-2-one | 110-93-0 | [33] | Strong, fatty, green, citrus-like | [36] |
| 2-Octanone | 111-13-7 | [33] | Floral, bitter-green, musty-herbaceous, unripe-apple fruity | [34,36] |
| 3-Octen-2-one | 1669-44-9 | [33] | Fruity, lemon | [36] |
| 2-Nonanone | 821-55-6 | [33] | Fruity-floral, fatty, herbaceous, rue | [36,60] |
| 3-Nonen-2-one | 14309-57-0 | Fruity | [36] | |
| Cyclics | ||||
| 2-Cyclopenten-1-one | 930-30-3 | [33] | - | |
| Cyclohexanone | 108-94-1 | Peppermint, acetone-like | [36] | |
| 4-Cyclopentene-1,3-dione | 930-60-9 | [20,63] | - | |
| 2-Methyl-2-cyclopenten-1-one | 1120-73-6 | [33] | - | |
| 2-Cyclohexen-1-one | 930-68-7 | [33] | Gassy-mint | [34] |
| 5-Ethylcyclopent-2-en-1-one | 34094-63-8 | - | ||
| 6-Methylenebicyclo[3.2.0]hept-3-en-2-one | - | - | ||
| 3-Methyl-2-cyclohexen-1-one | 1193-18-6 | [33] | Caramel-like, phenolic, mild cherry | [36,60] |
| 2,2,6-Trimethylcyclohexanone | 2408-37-9 | - | ||
| 2-Cyclohexene-1,4-dione | 4505-38-8 | - | ||
| 3,5-Dimethyl-2-cyclohexen-1-one | 1123-09-7 | - | ||
| 2-Hydroxy-3-methyl-2-cyclopenten-1-one | 80-71-7 | [33] | Sweet, caramel-like-spicy, walnut, maple, licorice, celery, tobacco | [34,35,52] |
| 2,3,4-Trimethyl-2-cyclopenten-1-one | 28790-86-5 | [29] | - | |
| 3,5-Dimethyl-1,2-cyclopentanedione | 13494-07-0 | [33] | - | |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 21835-01-8 | [33] | Caramel-like, sweet, sugary | [36,60] |
| Aromatics | ||||
| Acetophenone | 98-86-2 | [33] | Sweet | [36] |
| 1-Phenyl-2-propanone | 103-79-7 | - | ||
| o-Hydroxyacetophenone | 118-93-4 | [33] | Sweet, heavy-floral, herbaceous, new-mown hay-like, mimosa-like | [34] |
| 1-Phenyl-1,2-propanedione | 579-07-7 | [33] | Warm-floral, herbaceous, plastic | [34,36] |
| p-Methylacetophenone | 122-00-9 | Fruity, floral | [36] | |
| 1-Phenyl-2-butanone | 1007-32-5 | - | ||
| 1-(4-Hydroxyphenyl)-1-propanone | 70-70-2 | - | ||
| 4-Hydroxy-3-methylacetophenone | 876-02-8 | [64] | - | |
| Volatile phenols | ||||
| 2-methoxyphenol (Guaiacol) | 90-05-1 | [33] | Smoke-like, phenolic, burnt, spicy, woody, meaty, sweet | [20,35,36,39,44,60] |
| 2,6-Dimethylphenol | 576-26-1 | [33] | Ground-coffee, phenolic | [50,60] |
| 2-Allylphenol | 1745-81-9 | - | ||
| 4-Ethyl-2-methoxyphenol (4-Ethylguaiacol) | 2785-89-9 | [33] | Smoky, clove-like, spicy, burnt, vanilla-like, sweet, ethereal, green | [20,36,39,44,60] |
| 2-Methoxy-4-vinylphenol (4-Vinylguaiacol) | 7786-61-0 | [33] | Spicy, clove-like, phenolic, apple, rum, roasted peanut | [20,34,36,39,44,60] |
| Oxazoles | ||||
| 4-Methyloxazole | 693-93-6 | - | ||
| 4,5-Dimethyloxazole | 20662-83-3 | [33] | - | |
| Trimethyloxazole | 20662-84-4 | [33] | - | |
| 4-Ethyl-2,5-dimethyloxazole | 30408-61-8 | [33] | - | |
| 2-Ethyl-4-methyl-5-propyloxazole | 102586-53-8 | - | ||
| 4,5-Dimethyl-2-propyloxazole | 53833-32-2 | [33] | - | |
| 4,5-Dimethyl-2-isobutyloxazole | 26131-91-9 | - | ||
| Benzoxazole | 273-53-0 | [33] | - | |
| 2-Methylbenzoxazole | 95-21-6 | [33] | Sweet, gassy-pungent, floral-sweet, tobacco | [34,60] |
| Pyrazines | ||||
| Pyrazine | 290-37-9 | [33] | Pungent, sweet, floral, coffee | [20,34,54] |
| Methylpyrazine | 109-08-0 | [33] | Nutty, cocoa, green, roasted, chocolate, meaty, toasted | [20,34,54] |
| 2,5-Dimethylpyrazine | 123-32-0 | [33] | Chocolate, roasted nuts, earthy, grassy, roasted, nutty | [36,54,60] |
| 2-Ethylpyrazine | 13925-00-3 | [33] | Peanut butter, musty, nutty, woody, buttery, roasted, green, sweet | [36,60] |
| 2,3-Dimethylpyrazine | 5910-89-4 | [33] | Nutty, cocoa-like odor, green note, toasted, roasted | [20,36,54] |
| Vinylpyrazine | 4177-16-6 | [33] | - | |
| 2-Isopropylpyrazine | 9820-90-0 | - | ||
| 2-Ethyl-6-methylpyrazine | 13925-03-6 | [33] | Toasted, flowery, fruity, hazelnut-like | [2,20,54] |
| 2-Ethyl-3-methylpyrazine | 15707-23-0 | [33] | Raw-potato, roasted, earthy, nutty, peanut-like, coffee-like | [20,36,54] |
| 2-Propylpyrazine | 18138-03-9 | [33] | Green, vegetable, herbal | [2,36] |
| 2-Vinyl-6-methylpyrazine | 13925-09-2 | [33] | Coffee | [20] |
| Acetylpyrazine | 22047-25-2 | [33] | Toasted | [20] |
| 2-Methyl-3-isopropylpyrazine | 15986-81-9 | - | ||
| Isobutylpyrazine | 29460-92-2 | - | ||
| Isopropenylpyrazine | 38713-41-6 | [33] | - | |
| 2,6-Diethylpyrazine | 13067-27-1 | [33] | Toasted, potato-like, roasted | [2,20,54] |
| 2-Isopropyl-3-methoxypyrazine | 25773-40-4 | [33] | Vegetable-like, earthy, bell pepper, raw potato, galbanum, roasty, peasy | [36,38,39,60] |
| 6,7-Dihydro-5H-cyclopentapyrazine | 23747-47-9 | [33] | Green, phenolic, nutty, roast | [35,60] |
| 2-Acetyl-3-methylpyrazine | 23787-80-6 | [33] | Cereal, roasted bean, roasted, nutty, grain-roasted potato | [36,60] |
| 2-Isobutyl-3-methylpyrazine | 13925-06-9 | [33] | Herbaceous green earthy notes, green bell peppers notes | [36] |
| 5H-5-Methyl-6,7-dihydrocyclopentapyrazine | 23747-48-0 | [33] | Earthy, baked potato, peanut, roasted, nutty | [35,36,60] |
| 2-Methyl-6-(1-propenyl)pyrazine (isomer) | 104638-11-1 | [33] | - | |
| 2,3-Diethyl-5-methylpyrazine | 18138-04-0 | [33] | Nutty, meaty, roasted hazelnut, earthy, roasty | [35,36,44,45,52] |
| 2-Methyl-6-(1-propenyl)pyrazine (isomer) | 104638-11-1 | [33] | - | |
| 2-Acetyl-3-ethylpyrazine | 32974-92-8 | [29] | - | |
| 2-Isoamylpyrazine | 40790-22-5 | - | ||
| 2-Isobutyl-3-methoxypyrazine | 24683-00-9 | [33] | Green bell-pepper note, galbanum oil, red pepper, green, earthy | [4,36,44,60] |
| 2-Butyl-3-methylpyrazine | 15987-00-5 | [33] | - | |
| 2,5-Dimethyl-3-isobutylpyrazine | 32736-94-0 | [33] | - | |
| 1-Methylpyrrolo(1,2-a)pyrazine | 64608-59-9 | [33] | - | |
| 6,7-Dihydro-2,5-dimethyl-5H-cyclopentapyrazine | 38917-61-2 | [33] | - | |
| 2,5-Diethyl-3,6-dimethylpyrazine | 18903-30-5 | [33] | - | |
| 2-Methyl-6-isopentylpyrazine | 91010-41-2 | - | ||
| 2,6-Dimethyl-3(2-methyl-1-butyl)pyrazine | 56617-70-0 | - | ||
| 2,5-Dimethyl-3-isoamylpyrazine | 18433-98-2 | - | ||
| 2,3-Dimethyl-5-isopentylpyrazine | 18450-01-6 | - | ||
| Pyridines | ||||
| Pyridine | 110-86-1 | [33] | Pungent, nauseating, warm, burnt, smoky, coffee-like | [20,34,36,54] |
| 2-Methylpyridine | 109-06-8 | [33] | Roasted popcorn, coffee | [20,60] |
| 2,6-Dimethylpyridine | 108-48-5 | [33] | Minty-tarry, pyridine, peppermint | [36] |
| 2-Ethylpyridine | 100-71-0 | [33] | - | |
| 3-Ethylpyridine | 536-78-7 | [33] | Tobacco, caramel, burnt, coffee-like, toasted | [20,36,54,60] |
| 3-Vinylpyridine | 1121-55-7 | - | ||
| 2-Acetylpyridine | 1122-62-9 | [33] | Popcorn, bready, tobacco, cracker-like, roasted barley | [36,60] |
| Methyl 3-pyridinecarboxylate | 93-60-7 | [33] | Nauseating, sweet-herbaceous, mildly tobacco-like, fresh, caramel nutty | [34,36] |
| 2-Pentylpyridine | 2294-76-0 | Tallowy-like | [36] | |
| Pyrroles | ||||
| 1-Methylpyrrole | 96-54-8 | [33] | Smoky-tarry, sweet, woody-herbaceous, animal, coffee | [20,34] |
| Pyrrole | 109-97-7 | [33] | Warm, slightly pungent, hay-like herbaceous, sweet, green, toasted | [20,34,36,54] |
| 1-Ethylpyrrole | 617-92-5 | [33] | - | |
| 2,5-Dimethylpyrrole | 625-84-3 | [33] | - | |
| 2-Ethyl-4-methylpyrrole | 69687-77-0 | [37] | - | |
| 1-Acetylpyrrole | 609-41-6 | [33] | - | |
| 3-Ethyl-2,4-dimethylpyrrole | 517-22-6 | [33] | - | |
| 1-Methyl-2-formylpyrrole | 1192-58-1 | [33] | Cracker-popcorn, burnt | [54,60] |
| 1-Ethyl-2-formylpyrrole | 2167-14-8 | [33] | - | |
| 2-Acetyl-1-methylpyrrole | 932-16-1 | [33] | - | |
| 2-Acetylpyrrole | 1072-83-9 | [33] | Bread, walnut, licorice, cracker, popcorn-like | [20,36,60] |
| Sulfur compounds | ||||
| Dimethyl disulfide | 624-92-0 | [33] | Onion | [34,36] |
| Methylthio-2-propanone | 14109-72-9 | Melon | [36] | |
| 2-Furylmethylsulfide | 13129-38-9 | [33] | Garlic-like | [60] |
| 2-Furfurylthiol | 98-02-2 | [33] | Coffee-like, burnt-caramel-like, sweet, roasty, sulfury | [4,34,39,44,54] |
| 3-(Methylthio)propanal (Methional) | 3268-49-3 | [33] | Onion, meat-like, bouillon-like, soup-like, cooked potato-like | [4,34,36,38,46] |
| 1-(Methylthio)-2-butanone | 13678-58-5 | [33] | Mushroom, garlic | [36,60] |
| Dimethyl trisulfide | 3658-80-8 | [33] | Fresh onion, cabbage-like, brothy, sulfury, pungent | [4,34,36,37] |
| 3-Mercapto-3-methyl-1-butanol | 34300-94-2 | [33] | Sweet, soup-like, cooked meat, spicy, smoke-roast, meat, chicken brothy | [37,52,60] |
| 2-Furfuryl methyl sulfide | 1438-91-1 | [33] | Coffee-like, onion, garlic, burnt, sulfury, cooked cabbage | [20,36,37,60] |
| 3-Mercapto-3-methylbutyl formate | 50746-10-6 | [33] | Sweaty, fruity, blackcurrant-like, catty, orange flowers, roasty | [4,39,44,52,60] |
| 3-Mercapto-3-methylbutyl acetate | 50746-09-3 | [65] | - | |
| Furfuryl methyl disulfide | 57500-00-2 | [33] | Fresh white bread crust | [60] |
| Terpenic compounds | ||||
| Monoterpenes | ||||
| α-Pinene | 80-56-8 | [19] | Pine, turpentine-like | [36] |
| β-Pinene | 127-91-3 | [19] | Turpentine, dry, woody, resinous | [36] |
| 2,6-Dimethyl-2,6-octadiene (isomer) | 2792-39-4 | - | ||
| β-Myrcene | 123-35-3 | [33] | Sweet, balsamic, plastic, sweet-balsamic-resinous gum | [34,36] |
| 2,6-Dimethyl-2,6-octadiene (isomer) | 2792-39-4 | - | ||
| α-Phellandrene | 99-83-2 | [66] | Fresh, citrus, peppery, discrete mint, minty, herbaceous note | [36] |
| α-Terpinene | 99-86-5 | [19] | Woody, terpene, lemon | [36] |
| p-Cymene | 99-87-6 | [33] | Carrot-like, kerosene-like | [34,36] |
| Limonene | 138-86-3 | [33] | Citrusy, lemon-like, fresh, sweet | [60] |
| β-Ocimene (isomer) | 13877-91-3 | [49] | Warm herbaceous | [36] |
| β-Ocimene (isomer) | 13877-91-3 | [49] | Warm herbaceous | [36] |
| γ-Terpinene | 99-85-4 | [19] | Lemon | [36] |
| α-Terpinolene | 586-62-9 | Sweet, pine | [36] | |
| p-Cymenene | 1195-32-0 | [33] | Citrusy-lemon-like, gassy | [34] |
| Cosmene | 460-01-5 | - | ||
| Allo-ocimene | 673-84-7 | - | ||
| Monoterpenoids | ||||
| Linalool oxide (isomer) | 1365-19-1 | [33] | Sweet, woody, floral, woody-earthy undertone, pungent | [34,36] |
| Linalool oxide (isomer) | 1365-19-1 | [33] | Sweet, woody, floral, woody-earthy undertone, pungent | [34,36] |
| Linalool | 78-70-6 | [33] | Floral-woody, faintly citrusy note, floral, sweet-fruity | [34,35,36,39,50,52] |
| α-Terpineol | 98-55-5 | [33] | Floral, lilac | [60] |
| Safranal | 116-26-7 | Saffron-like | [36] | |
| p-Menth-1-en-9-al | 29548-14-9 | - | ||
| Sesquiterpenes | ||||
| α-Cubebene | 17699-14-8 | - | ||
| α-Copaene | 3856-25-5 | [29] | - | |
| Longifolene | 475-20-7 | - | ||
| β-Caryophyllene | 87-44-5 | Cloves, turpentine | [36] | |
| α-Humulene | 6753-98-6 | - | ||
| α-Muurolene | 31983-22-9 | - | ||
| δ-Cadinene | 483-76-1 | [29] | - | |
| Norisoprenoids | ||||
| Vitispirane (C13) | 65416-59-3 | - | ||
| Theaspirane (C13) | 36431-72-8 | Fruity, woody, sweetish | [36] | |
| 1,2-Dihydro-1,1,6-trimethylnaphthalene (C13) | 30364-38-6 | - | ||
| β-Damascenone (C13) | 23726-93-4 | [33] | Tea-like, fruity, honey-like, fruity, sweet-fruity | [2,39,44,50,52,60,62] |
| α-Ionone (C13) | 127-41-3 | Warm, woody, berry characteristic violet-like | [36] | |
| Geranyl acetone (C13) | 689-67-8 | Green, rosy floral, fresh-floral, sweet-rosy, slightly green magnolia-like | [36] | |
| Thiazoles | ||||
| Thiazole | 288-47-1 | [33] | Green, sweet, nutty, tomato, toasted | [20,36] |
| 2-Methylthiazole | 3581-87-1 | [33] | - | |
| 4-Methylthiazole | 693-95-8 | [33] | Nutty, green, roasted | [36,54] |
| 5-Methylthiazole | 3581-89-3 | [33] | - | |
| 2,4-Dimethylthiazole | 541-58-2 | [33] | Salty, sulfury, burnt, rubber | [37] |
| 2,5-Dimethylthiazole | 4175-66-0 | [33] | - | |
| 4,5-Dimethylthiazole | 3581-91-7 | [33] | Roasted nutty, boiled poultry | [36] |
| 5-Ethylthiazole | 17626-73-2 | [33] | - | |
| 2-Ethyl-4-methylthiazole | 15679-12-6 | [33] | Nutty, green | [36] |
| 4-Propylthiazole | 41981-60-6 | - | ||
| 5-Ethyl-2-methylthiazole | 19961-52-5 | [33] | Rubber-like | [2] |
| 2-Isopropyl-4-methylthiazole | 15679-13-7 | Green, vegetable, nutty, rooty, earthy | [36] | |
| 5-Ethyl-4-methylthiazole | 31883-01-9 | [33] | Nutty, green, earthy | [60] |
| 2-Acetylthiazole | 24295-03-2 | [33] | Green onion, herbal, grassy | [36] |
| 4-Ethyl-2,5-dimethylthiazole | 32272-57-4 | [33] | - | |
| 5-Ethyl-2,4-dimethylthiazole | 38205-61-7 | [33] | Earthy, roasty | [39] |
| 2-Acetyl-4-methylthiazole | 7533-07-5 | [33] | - | |
| 2-Propanoyl-thiazole | 43039-98-1 | - | ||
| Benzothiazole | 95-16-9 | [33] | Delicate, persistent, rose-like | [36] |
| Tiophene compounds | ||||
| Thiophene | 110-02-1 | [33] | - | |
| 2-Methylthiophene | 554-14-3 | [33] | Onion, sulfury | [60] |
| 3-Methylthiophene | 616-44-4 | [33] | - | |
| 2-Ethylthiophene | 872-55-9 | [47,49] | - | |
| 2,5-Dimethylthiophene | 638-02-8 | [37] | - | |
| 2,4-Dimethylthiophene | 638-00-6 | - | ||
| 2,3-Dimethylthiophene | 632-16-6 | [37] | - | |
| 2-Vinylthiophene | 1918-82-7 | [37] | - | |
| 3-Methoxythiophene | 17573-92-1 | - | ||
| 3-Thiophanone | 1003-04-9 | [33] | Garlic meaty, green vegetable, buttery | [36] |
| 2-Isopropylthiophene | 4095-22-1 | - | ||
| 2,3,4-Trimethylthiophene | 1795-04-6 | - | ||
| Dihydro- 2-methyl-3(2H)-thiophenone | 13679-85-1 | [33] | - | |
| 3-Thiophenecarboxaldehyde | 498-62-4 | - | ||
| Dihydro-2(3H)-thiophenone | 1003-10-7 | [33] | - | |
| 2-Thiophenecarboxaldehyde | 98-03-3 | [33] | Coffee | [20] |
| 3-Methyl-2-thiophenecarboxaldehyde | 5834-16-2 | [33] | - | |
| 3-Acetylthiophene | 1468-83-3 | [33] | - | |
| 2-Acetylthiophene | 88-15-3 | [33] | - | |
| 2,5-Diethylthiophene | 5069-23-8 | - | ||
| Methyl-2-thiophene carboxylate | 5380-42-7 | [33] | - | |
| 5-Methyl-2-thiophenecarboxaldehyde | 13679-70-4 | [33] | - | |
| 2-Pentylthiophene | 4861-58-9 | - | ||
| 2-Propionylthiophene | 13679-75-9 | [33] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, G.R.; Petronilho, S.; Ferreira, A.S.; Pinto, M.; Passos, C.P.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.M.; Coimbra, M.A. Insights on Single-Dose Espresso Coffee Capsules’ Volatile Profile: From Ground Powder Volatiles to Prediction of Espresso Brew Aroma Properties. Foods 2021, 10, 2508. https://doi.org/10.3390/foods10102508
Lopes GR, Petronilho S, Ferreira AS, Pinto M, Passos CP, Coelho E, Rodrigues C, Figueira C, Rocha SM, Coimbra MA. Insights on Single-Dose Espresso Coffee Capsules’ Volatile Profile: From Ground Powder Volatiles to Prediction of Espresso Brew Aroma Properties. Foods. 2021; 10(10):2508. https://doi.org/10.3390/foods10102508
Chicago/Turabian StyleLopes, Guido R., Sílvia Petronilho, Andreia S. Ferreira, Mariana Pinto, Claúdia P. Passos, Elisabete Coelho, Carla Rodrigues, Cláudia Figueira, Sílvia M. Rocha, and Manuel A. Coimbra. 2021. "Insights on Single-Dose Espresso Coffee Capsules’ Volatile Profile: From Ground Powder Volatiles to Prediction of Espresso Brew Aroma Properties" Foods 10, no. 10: 2508. https://doi.org/10.3390/foods10102508