Antioxidant and Immunostimulatory Activities of a Submerged Culture of Cordyceps sinensis Using Spent Coffee
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Defatted SCG and Submerged Culture of C. sinensis
2.3. Analytical Methods
2.4. Assay of Radical Scavenging Activity
2.5. Preparation of the EPS Fraction
2.6. Assay for Cytokine Release from Peritoneal Macrophages
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Reducing Sugar, Glucosamine, and Polyphenol Contents in a Submerged Culture of C. sinensis with SCG Using an 8 L Fermenter
3.2. Total Sugar, Reducing Sugar, Uronic Acid, and CQA Contents of SCG Fermented Using Submerged Culture of C. sinensis
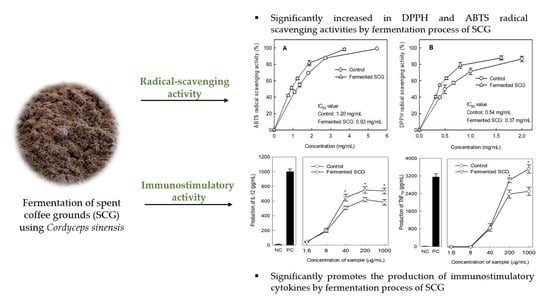
3.3. Antioxidant Activity of Fermented SCG
3.4. Chemical Properties and Immune-Stimulating Activity of EPS Obtained from SCG in Submerged Culture of C. sinensis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Wang, S.; Rupasinghe, H.V. Experimental exploration of processes for deriving multiple products from spent coffee grounds. Food Bioprod. Process. 2021, 128, 21–29. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Alsanad, M.A.; Sassine, Y.N.; El Sebaaly, Z.; Abou Fayssal, S. Spent coffee grounds influence on Pleurotus ostreatus production, composition, fatty acid profile, and lignocellulose biodegradation capacity. CYTA J. Food 2021, 19, 11–20. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Jeganathan, P.M. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Simoes, J.; Madureira, P.; Nunes, F.M.; Domingues, M.D.; Vilanova, M.; Coimbra, M.A. Immunostimulatory properties of coffee mannans. Mol. Nutr. Food Res. 2009, 53, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Chi, H.; Ji, G.E. Transformation of ginsenosides Rb1 and Re from P anax ginseng by food microorganisms. Biotechnol. Lett. 2005, 27, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, Y.S.; Ra, K.S.; Suh, H.J. Characteristics of fermentation and bioavailability of isoflavones in Korean soybean paste (doenjang) with application of Bacillus sp. KH-15. Int. J. Food Sci. Technol. 2007, 42, 1497–1503. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Illana, E.C. Cordyceps sinensis, a fungi used in the Chinese traditional medicine. Rev. Iberoam. Micol. 2007, 24, 259–262. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Won, D.P.; Lee, J.H.; Lee, K.E.; Lee, S.Y.; Hong, E.K. Study of macrophage activation and structural characteristics of purified polysaccharide from the fruiting body of Cordyceps militaris. J. Microbiol. Biotechnol. 2010, 20, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Park, E.D.; Park, Y.; Suh, H.J. Spent coffee ground extract suppresses ultraviolet B-induced photoaging in hairless mice. J. Photoch. Photobio. B 2015, 153, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Blumenkr, N.; Asboehan, G. New Method for Quantitative-Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Swift, M. The estimation of mycelial biomass by determination of the hexosamine content of wood tissue decayed by fungi. Soil Biol. Biochem. 1973, 5, 321–332. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Jones, T.M.; Albersheim, P. A gas chromatographic method for the determination of aldose and uronic acid constituents of plant cell wall polysaccharides. Plant Physiol. 1972, 49, 926–936. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.F.; Kiyohara, H.; Yamada, H.; Takemoto, N.; Kawamura, H. Heterogeneity and characterisation of mitogenic and anti-complementary pectic polysaccharides from the roots of Glycyrrhiza uralensis Fisch et D.C. Carbohydr. Res. 1991, 219, 149–172. [Google Scholar] [CrossRef]
- Jo, K.; Kim, S.; Ahn, Y.; Suh, H.J. Effects of green lettuce leaf extract on sleep disturbance control in oxidative stress-induced invertebrate and vertebrate models. Antioxidants 2021, 10, 970. [Google Scholar] [CrossRef]
- Moon, J.K.; Yoo, H.S.; Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef]
- Quang, D.N.; Hashimoto, T.; Nukada, M.; Yamamoto, I.; Tanaka, M.; Asakawa, Y. Antioxidant activity of curtisians I-L from the inedible mushroom Paxillus curtisii. Planta Med. 2003, 69, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Xiong, Y.L.L. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. J. Agric. Food. Chem. 2005, 53, 9186–9192. [Google Scholar] [CrossRef]
- Lee, H.S.; Won, N.H.; Kim, K.H.; Lee, H.; Jun, W.; Lee, K.W. Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol. Pharm. Bull. 2005, 28, 1639–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, I.; Tanaka, H.; Kinoshita, A.; Oikawa, S.; Osawa, M.; Yadomae, T. Effect of orally administered β-glucan on macrophage function in mice. Int. J. Immunopharmacol. 1990, 12, 675–684. [Google Scholar] [CrossRef]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine metabolism during cultivation of oyster mushroom (Pleurotus ostreatus) with spent coffee grounds. Appl. Microbiol. Biot. 2019, 103, 5831–5841. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Ranamukhaarachchi, S.L. Study on the mycelium growth and primordial formation of king oyster mushroom (Pleurotus eryngii) on cardboard and spent coffee ground. Res. Crop. 2019, 20, 835–842. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohyd. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.-H.; Yu, K.-W.; Suh, H.-J.; Choi, Y.-M.; Ahn, T.-S. Activation of macrophages and the intestinal immune system by an orally administered decoction from cultured mycelia of Cordyceps sinensis. Biosci. Biotechnol. Biochem. 2002, 66, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, T.J.; Yu, K.W.; Shin, K.S.; Suh, H.J. Innate immune stimulation of exo-polymers prepared from Cordyceps sinensis by submerged culture. Appl. Microbiol. Biot. 2008, 80, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Parras, P.; Martinez-Tome, M.; Jimenez, A.M.; Murica, M.A. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem. 2007, 102, 582–592. [Google Scholar] [CrossRef]
- Corso, M.P.; Vignoli, J.A.; Benassi, M.D. Development of an instant coffee enriched with chlorogenic acids. J. Food Sci. Technol. 2016, 53, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Benitez, J. CYP1A2 activity, gender and smoking, as variables influencing the toxicity of caffeine. Brit. J. Clin. Pharmacol. 1996, 41, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Agudelo-Ochoa, G.M.; Pulgarin-Zapata, I.C.; Velasquez-Rodriguez, C.M.; Duque-Ramirez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzman, O.J.; Munoz-Durango, K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Lei, Z.F.; Lu, Y.; Lu, Z.Z.; Chen, Y. Chemical composition and bioactivity changes in stale rice after fermentation with Cordyceps sinensis. J. Biosci. Bioeng. 2008, 106, 188–193. [Google Scholar] [CrossRef]
- Lateef, A.; Oloke, J.K.; Kana, E.B.G.; Oyeniyi, S.O.; Onifade, O.R.; Oyeleye, A.O.; Oladosu, O.C.; Oyelami, A.O. Improving the quality of agro-wastes by solid-state fermentation: Enhanced antioxidant activities and nutritional qualities. World. J. Microb. Biot. 2008, 24, 2369–2374. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyota, S.; Renard, C.M.G.C. Interactions between apple (Malus x domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohyd. Polym. 2009, 75, 251–261. [Google Scholar] [CrossRef]
- Machado, E.M.; Rodriguez-Jasso, R.M.; Teixeira, J.A.; Mussatto, S.I. Growth of fungal strains on coffee industry residues with removal of polyphenolic compounds. Biochem. Eng. J. 2012, 60, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A Theoretical basis for a biopharmaceutic drug classification—The correlation of in-vitro drug product dissolution and in-vivo bioavailability. Pharmaceut. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Liyana-Pathlrana, C.M.; Shahidi, F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem. 2007, 101, 1151–1157. [Google Scholar] [CrossRef]
- Dumville, J.C.; Fry, S.C. Uronic acid-containing oligosaccharins: Their biosynthesis, degradation and signalling roles in non-diseased plant tissues. Plant Physiol. Biochem. 2000, 38, 125–140. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shitara, E.; Adachi, H.; Toyoshima, M.; Nakajima, M.; Okami, Y.; Takeuchi, T. Flexible synthesis and biological activity of uronic acid-type gem-diamine 1-N-imnosugars: A new family of glycosidase inhibitors. J. Org. Chem. 2000, 65, 2–11. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.N.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762. [Google Scholar] [CrossRef]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.C.; Chang, C.Y.; Cheng, T.L.; Wu, M.J. Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: Enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl. Microbiol. Biot. 2007, 75, 769–775. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.; Hessenkemper, W.; Schaper, F. Systems biology of IL-6, IL-12 family cytokines. Cytokine Growth Factor Rev. 2015, 26, 595–602. [Google Scholar] [CrossRef]
- Zheng, H.; Ban, Y.; Wei, F.; Ma, X.J. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Exp. Med. Biol. 2016, 941, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Newport, M.J.; Holland, S.M.; Levin, M.; Casanova, J.-L. Inherited disorders of the interleukin-12/23-interferon gamma axis. In Primary Immunodeficiency Diseases: A Molecular and Genetic Approach; Ochs, H.D., Smith, C.I., Puck, J., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 390–401. [Google Scholar]
- Hendy, P.A.; Reddi, D.; Bernardo, D.; Durant, L.; Noble, A.; English, N.R.; Knight, S.C.; Hart, A. Aberrant circulating dendritic cell cytokine production and homing profile in Crohn’s disease is normalised by anti-TNF-alpha therapy. Gastroenterology 2016, 150, S817–S818. [Google Scholar] [CrossRef]



| Sample | Total Sugar (mg/g dw) | Reducing Sugar (mg/g dw) | Uronic Acid (mg/g dw) | Total Polyphenol (mg GAE/g dw) |
|---|---|---|---|---|
| Control | 448.54 ± 17.78 | 423.11 ± 10.57 | 7.81 ± 0.52 | 55.04 ± 1.61 |
| Fermented SCG | 396.27 ± 42.14 * | 217.26 ± 5.37 * | 11.74 ± 0.30 * | 81.18 ± 1.38 * |
| Sample | Content (mg/g dw) | |||
|---|---|---|---|---|
| 3,4-Dihydrobenzoic Acid | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | |
| Control | 2.90 ± 0.17 | 1.84 ± 0.03 | 0.074 ± 0.001 | 3.65 ± 0.06 |
| Fermented SCG | 0.63 ± 0.04 *** | 5.04 ± 0.18 *** | 0.10 ± 0.004 ** | 6.52 ± 0.16 *** |
| Sample | Rutin | Ellagic acid | Quercetin | Kaempferol |
| Control | 0.49 ± 0.003 | 0.22 ± 0.002 | 0.56 ± 0.009 | n.d. |
| Fermented SCG | 0.66 ± 0.01 *** | 0.82 ± 0.007 *** | 2.74 ± 0.21 *** | 0.49 ± 0.006 *** |
| Sample | Chlorogenic Acid (mg/g dw) | Caffeine(mg/g dw) | ||
|---|---|---|---|---|
| 3-CQA | 4-CQA | 5-CQA | ||
| Control | 1.15 ± 0.04 | 0.63 ± 0.01 | 0.74 ± 0.02 | 15.18 ± 0.04 |
| Fermented SCG | 4.15 ± 0.06 *** | 2.26 ± 0.02 *** | 2.71 ± 0.01 *** | 29.32 ± 0.01 *** |
| Coffee Spent (30 g) | Control | Fermented SCG |
|---|---|---|
| Chemical composition | Mol% | |
| Neutral sugar | 88.6 ± 6.2 | 91.9 ± 1.9 |
| KDO-liked material | n.d. | n.d. |
| Protein | 4.1 ± 1.4 | n.d. |
| Sugar component | Mol% | |
| Rhamnose | 0.1 ± 0.1 | 0.3 ± 0.1 |
| Fucose | n.d. | n.d. |
| Arabinose | 2.2 ± 0.1 | 3.1 ± 0.5 * |
| Xylose | n.d. | n.d. |
| Mannose | 47.8 ± 0.5 | 56.5 ± 1.7 ** |
| Galactose | 22.4 ± 0.6 | 23.6 ± 2.2 |
| Glucose | 16.2 ± 0.2 | 8.4 ± 0.1 *** |
| GalA + GluA | 7.0 ± 0.3 | 7.6 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.H.; Ahn, Y.; Lee, H.J.; Suh, H.J.; Jo, K. Antioxidant and Immunostimulatory Activities of a Submerged Culture of Cordyceps sinensis Using Spent Coffee. Foods 2021, 10, 1697. https://doi.org/10.3390/foods10081697
Han SH, Ahn Y, Lee HJ, Suh HJ, Jo K. Antioxidant and Immunostimulatory Activities of a Submerged Culture of Cordyceps sinensis Using Spent Coffee. Foods. 2021; 10(8):1697. https://doi.org/10.3390/foods10081697
Chicago/Turabian StyleHan, Sung Hee, Yejin Ahn, Hyun Jung Lee, Hyung Joo Suh, and Kyungae Jo. 2021. "Antioxidant and Immunostimulatory Activities of a Submerged Culture of Cordyceps sinensis Using Spent Coffee" Foods 10, no. 8: 1697. https://doi.org/10.3390/foods10081697