Low Oxygen Storage Improves Tomato Postharvest Cold Tolerance, Especially for Tomatoes Cultivated with Far-Red LED Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Setup
2.3. CA storage Conditions
2.4. Respiration Measurements
2.5. Colour and Firmness Measurement
2.6. Disorder Index and Weight Loss
2.7. Hydrogen Peroxide (H2O2) Measurement
2.8. Statistical Analysis
3. Results
3.1. Experiment 1: Effects of Low Oxygen Conditions on CI Indices, Weight- and Firmness Loss
3.2. Experiment 1: Effects of Low Oxygen Conditions on Tomato Colour Development
3.3. Experiment 2: Effects of Low Oxygen Storage of Mature Green Tomatoes Cultivated with and without Far Red Lighting on CI Symptoms, Weight- and Firmness Loss
3.4. Effects of Low Oxygen Conditions on Colour Development of Mature Green Tomatoes Cultivated with and without Far Red Lighting
3.5. Effects of Low Oxygen Conditions on Respiration and H2O2 Production of Mature Green Tomatoes Cultivated with and without Far Red Lighting
4. Discussion
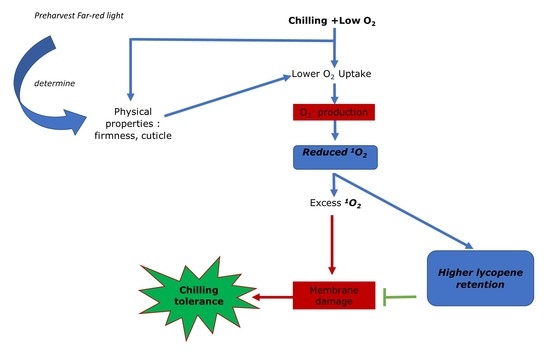
4.1. Low Oxygen Storage Alleviated CI in Tomato Which Might Be Related to Lower Oxygen Uptake and Improved Lycopene Synthesis
4.2. Low oxygen Storage Alleviated CI in Tomato Which Might Be Related to Lower Oxygen Uptake and Improved Lycopene Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albornoz, K.; Cantwell, M.I.; Zhang, L.; Beckles, D.M. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P. Oxidative Stress: Importance for Postharvest Quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, M.S.; Bodbodak, S. Postharvest Heat Treatment for Mitigation of Chilling Injury in Fruits and Vegetables. Food Bioprocess Technol. 2013, 7, 37–53. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Maul, F.; Sargent, S.; Sims, C.; Baldwin, E.; Balaban, M.; Huber, D. Tomato Flavor and Aroma Quality as Affected by Storage Temperature. J. Food Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Arginase induction by heat treatment contributes to amelioration of chilling injury and activation of antioxidant enzymes in tomato fruit. Postharvest Biol. Technol. 2013, 79, 1–8. [Google Scholar] [CrossRef]
- Pesis, E.; Aharoni, D.; Aharon, Z.; Ben-Arie, R.; Aharoni, N.; Fuchs, Y. Modified atmosphere and modified humidity packaging alleviates chilling injury symptoms in mango fruit. Postharvest Biol. Technol. 2000, 19, 93–101. [Google Scholar] [CrossRef]
- Singh, S.; Pal, R. Controlled atmosphere storage of guava (Psidium guajava L.) fruit. Postharvest Biol. Technol. 2008, 47, 296–306. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z. Controlled and modified atmospheres influence chilling injury, fruit quality and antioxidative system of Japanese plums (Prunus salicina Lindell). Int. J. Food Sci. Technol. 2012, 48, 363–374. [Google Scholar] [CrossRef]
- Alamar, M.C.; Collings, E.; Cools, K.; Terry, L.A. Impact of controlled atmosphere scheduling on strawberry and imported avocado fruit. Postharvest Biol. Technol. 2017, 134, 76–86. [Google Scholar] [CrossRef] [Green Version]
- de Almeida Teixeira, G.; Santos, L.; Cunha Júnior, L.; Durigan, J. Effect of carbon dioxide (CO2) and oxygen (O2) levels on quality of ‘Palmer’ mangoes under controlled atmosphere storage. J. Food Sci. Technol. 2018, 55, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Jin, M.; Guo, L.; Pei, H.; Nan, Y.; Rao, J. Modified atmosphere packaging and 1-methylcyclopropene alleviate chilling injury of ‘Youhou’ sweet persimmon during cold storage. Food Packag. Shelf Life 2020, 24, 100479. [Google Scholar] [CrossRef]
- Esanhueza, D.; Evizoso, P.; Ebalic, I.; Campos-Vargas, R.; Emeneses, C. Transcriptomic analysis of fruit stored under cold conditions using controlled atmosphere in Prunus persica cv. “Red Pearl”. Front. Plant Sci. 2015, 6, 788. [Google Scholar] [CrossRef]
- Beaudry, R.M. Responses of Horticultural Commodities to Low Oxygen: Limits to the Expanded Use of Modified Atmosphere Packaging. HortTechnology 2000, 10, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; Forney, C.F. The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 2000, 51, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Sabban-Amin, R.; Feygenberg, O.; Belausov, E.; Pesis, E. Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘Granny Smith’ apples. Postharvest Biol. Technol. 2011, 62, 295–304. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Aguilar, G.A.; Villa-Rodriguez, J.A.; Zavala, J.F.A.; Yahia, E.M. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends Food Sci. Technol. 2010, 21, 475–482. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.; Vries, F.; van der Merwe, K.; Crouch, E.; Opara, U.L. Impact of dynamic controlled atmospheres on reactive oxygen species, antioxidant capacity and phytochemical properties of apple peel (cv. Granny Smith). Sci. Hortic. 2017, 216, 169–176. [Google Scholar] [CrossRef]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, D.; Brizzolara, S.; Tonutti, P. Physiological and biochemical effects of controlled and modified atmospheres. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 425–441. [Google Scholar]
- Cukrov, D.; Zermiani, M.; Brizzolara, S.; Cestaro, A.; Licausi, F.; Luchinat, C.; Santucci, C.; Tenori, L.; Van Veen, H.; Zuccolo, A.; et al. Extreme Hypoxic Conditions Induce Selective Molecular Responses and Metabolic Reset in Detached Apple Fruit. Front. Plant Sci. 2016, 7, 146. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, B.D. Changes in lipids of tomato fruit stored at chilling and non-chilling temperatures. Phytochemistry 1991, 30, 757–761. [Google Scholar] [CrossRef]
- Lurie, S.; Sabehat, A. Prestorage temperature manipulations to reduce chilling injury in tomatoes. Postharvest Biol. Technol. 1997, 11, 57–62. [Google Scholar] [CrossRef]
- Biswas, P.; East, A.R.; Brecht, J.K.; Hewett, E.W.; Heyes, J. Intermittent warming during low temperature storage reduces tomato chilling injury. Postharvest Biol. Technol. 2012, 74, 71–78. [Google Scholar] [CrossRef]
- Affandi, F.Y.; Verdonk, J.C.; Ouzounis, T.; Ji, Y.; Woltering, E.J.; Schouten, R.E. Far-red light during cultivation induces postharvest cold tolerance in tomato fruit. Postharvest Biol. Technol. 2020, 159, 1–10. [Google Scholar] [CrossRef]
- Bulens, I.; Van De Poel, B.; Hertog, M.L.; De Proft, M.P.; Geeraerd, A.H.; Nicolaï, B.M. Protocol: An updated integrated methodology for analysis of metabolites and enzyme activities of ethylene biosynthesis. Plant Methods 2011, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Schouten, R.E.; Zhang, X.; Verschoor, J.A.; Otma, E.C.; Tijskens, P.; Van Kooten, O. Development of colour of broccoli heads as affected by controlled atmosphere storage and temperature. Postharvest Biol. Technol. 2009, 51, 27–35. [Google Scholar] [CrossRef]
- Schouten, R.E.; Farneti, B.; Tijskens, P.; Alarcón, A.A.; Woltering, E.J. Quantifying lycopene synthesis and chlorophyll breakdown in tomato fruit using remittance VIS spectroscopy. Postharvest Biol. Technol. 2014, 96, 53–63. [Google Scholar] [CrossRef]
- Schouten, R.E.; Fan, S.; Verdonk, J.C.; Wang, Y.; Kasim, N.F.M.; Woltering, E.J.; Tijskens, P. Mango Firmness Modeling as Affected by Transport and Ethylene Treatments. Front. Plant Sci. 2018, 9, 1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 05, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Klieber, A.; Ratanachinakorn, B.; Simons, D. Effects of low oxygen and high carbon dioxide on tomato cultivar ‘Bermuda’ fruit physiology and composition. Sci. Hortic. 1996, 65, 251–261. [Google Scholar] [CrossRef]
- Fahmy, K.; Nakano, K. The Individual and Combined Influences of Low Oxygen and High Carbon Dioxide on Chilling-injury Alleviation in Cucumber Fruit. Environ. Control. Biol. 2014, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Malacrida, C.; Valle, E.M.; Boggio, S.B. Postharvest chilling induces oxidative stress response in the dwarf tomato cultivar Micro-Tom. Physiol. Plant. 2006, 127, 10–18. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Martinez, A.; Stinco, C.M.; Melendez-Martinez, A.J. Free Radical Scavenging Properties of Phytofluene and Phytoene Isomers as Compared to Lycopene: A Combined Experimental and Theoretical Study. J. Phys. Chem. B 2014, 118, 9819–9825. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; López-Climent, M.; Gómez-Cadenas, A.; Zacarías, L. Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biol. Technol. 2016, 111, 214–223. [Google Scholar] [CrossRef]
- Farneti, B.; Schouten, R.E.; Woltering, E.J. Low temperature-induced lycopene degradation in red ripe tomato evaluated by remittance spectroscopy. Postharvest Biol. Technol. 2012, 73, 22–27. [Google Scholar] [CrossRef]
- Garstka, M.; Venema, J.H.; Rumak, I.; Gieczewska, K.; Rosiak, M.; Koziol-Lipinska, J.; Kierdaszuk, B.; Vredenberg, W.J.; Mostowska, A. Contrasting effect of dark-chilling on chloroplast structure and arrangement of chlorophyll–protein complexes in pea and tomato: Plants with a different susceptibility to non-freezing temperature. Planta 2007, 226, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, M.-R.; Zhao, Y.-Y.; Mao, L.-C. Reduction of Chilling Injury and Ultrastructural Damage in Cherry Tomato Fruits after Hot Water Treatment. Agric. Sci. China 2009, 8, 304–310. [Google Scholar] [CrossRef]
- Skupień, J.; Wójtowicz, J.; Łucja, K.; Mazur, R.; Garstka, M.; Gieczewska, K.; Mostowska, A. Dark-chilling induces substantial structural changes and modifies galactolipid and carotenoid composition during chloroplast biogenesis in cucumber (Cucumis sativus L.) cotyledons. Plant Physiol. Biochem. 2017, 111, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mirdehghan, S.H.; Rahemi, M.; Martínez-Romero, D.; Guillen, F.; Valverde, J.; Zapata, P.J.; Serrano, M.; Valero, D. Reduction of pomegranate chilling injury during storage after heat treatment: Role of polyamines. Postharvest Biol. Technol. 2007, 44, 19–25. [Google Scholar] [CrossRef]
- Rodoni, L.; Casadei, N.; Concellon, A.; Alicia, A.R.C.; Vicente, A. Effect of Short-Term Ozone Treatments on Tomato (Solanum lycopersicum L.) Fruit Quality and Cell Wall Degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Cozmuta, A.M.; Cozmuta, L.M.; Peter, A.; Nicula, C.; Vosgan, Z.; Giurgiulescu, L.; Vulpoi, A.; Baia, M. Effect of monochromatic Far-Red light on physical-nutritional-microbiological attributes of red tomatoes during storage. Sci. Hortic. 2016, 211, 220–230. [Google Scholar] [CrossRef]
- Bhowmik, S.R.; Pan, J.C. Shelf Life of Mature Green Tomatoes Stored in Controlled Atmosphere and High Humidity. J. Food Sci. 1992, 57, 948–953. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affandi, F.Y.; Verschoor, J.A.; Paillart, M.J.M.; Verdonk, J.C.; Woltering, E.J.; Schouten, R.E. Low Oxygen Storage Improves Tomato Postharvest Cold Tolerance, Especially for Tomatoes Cultivated with Far-Red LED Light. Foods 2021, 10, 1699. https://doi.org/10.3390/foods10081699
Affandi FY, Verschoor JA, Paillart MJM, Verdonk JC, Woltering EJ, Schouten RE. Low Oxygen Storage Improves Tomato Postharvest Cold Tolerance, Especially for Tomatoes Cultivated with Far-Red LED Light. Foods. 2021; 10(8):1699. https://doi.org/10.3390/foods10081699
Chicago/Turabian StyleAffandi, Fahrizal Yusuf, Jan A. Verschoor, Maxence J. M. Paillart, Julian C. Verdonk, Ernst J. Woltering, and Rob E. Schouten. 2021. "Low Oxygen Storage Improves Tomato Postharvest Cold Tolerance, Especially for Tomatoes Cultivated with Far-Red LED Light" Foods 10, no. 8: 1699. https://doi.org/10.3390/foods10081699