A New Way to Model Periodontitis in Laboratory Animals
Abstract
:1. Introduction
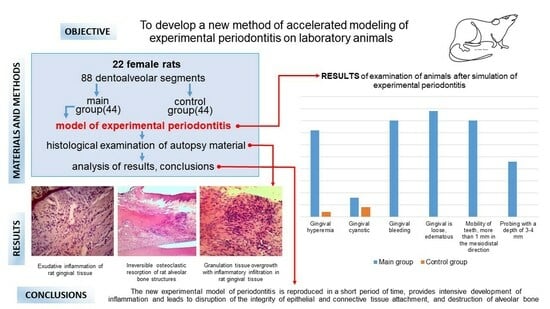
2. Materials and Methods
3. Results
3.1. Dental Examination
3.2. Histologic Study
4. Discussion
5. Conclusions
- The new model of periodontitis in rats allows us to consider the reaction of animal tissues at the histological and macroscopic level before exposure and at the peak of developed inflammation.
- The periodontitis model leads to intensive infiltration of rat periodontal tissues with inflammatory cells.
- The developed technique allows modeling periodontitis leading to disruption of epithelial and connective tissue attachment integrity, destruction of alveolar bone.
- The new model of periodontitis is reproduced in a short time (up to 7 days).
- The methodology of periodontitis modeling does not include the use of traumatic elements for animals and obviously stressful conditions of animal housing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchesan, J.; Girnary, M.S.; Jing, L.; Miao, M.Z.; Zhang, S.; Sun, L.; Morelli, T.; Schoenfisch, M.H.; Inohara, N.; Offenbacher, S.; et al. An experimental murine model to study periodontitis. Nat. Protoc. 2018, 13, 2247–2267. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Alawaji, Y.N.; Alshammari, A.; Mostafa, N.; Carvalho, R.M.; Aleksejuniene, J. Periodontal disease prevalence, extent, and risk associations in untreated individuals. Clin. Exp. Dent. Res. 2022, 8, 380–394. [Google Scholar] [CrossRef]
- Unanyan, K.G.; Balmasova, I.P.; Tsarev, V.N.; Mkrtumyan, A.M.; Elbekyan, K.S.; Karakov, K.G.; Gontarenko, M.S.; Arutyunov, S.D. Lipid metabolism as microecological and systemic factor in the development of periodontal disease: A review. Clin. Dent. 2020, 3, 36–43. (In Russian) [Google Scholar] [CrossRef]
- Oh, S.; Chung, S.H.; Han, J.-Y. Periodontal regenerative therapy in endo-periodontal lesions: A retrospective study over 5 years. J. Periodontal Implant Sci. 2019, 49, 90–104. [Google Scholar] [CrossRef]
- Li, D.; Qiu, Y.; Zhang, S.; Zhang, M.; Chen, Z.; Chen, J. A Multifunctional Antibacterial and Osteogenic Nanomedicine: QAS-Modified Core-Shell Mesoporous Silica Containing Ag Nanoparticles. Biomed. Res. Int. 2020, 2020, 4567049. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Hayashi, J.I.; Mitani, A. Next-Generation Examination, Diagnosis, and Personalized Medicine in Periodontal Disease. J. Pers. Med. 2022, 12, 1743. [Google Scholar] [CrossRef]
- Dzampaeva, Z.V.; Datieva, F.S.; Ephiev, A.A.; Dzaraeva, L.B. Method of Modeling Periodontitis in Experiment. Patent RU 2 699 497 C2, 5 September 2019. (In Russian). [Google Scholar]
- Rojas, C.; García, M.P.; Polanco, A.F.; González-Osuna, L.; Sierra-Cristancho, A.; Melgar-Rodríguez, S.; Cafferata, E.A.; Vernal, R. Humanized Mouse Models for the Study of Periodontitis: An Opportunity to Elucidate Unresolved Aspects of Its Immunopathogenesis and Analyze New Immunotherapeutic Strategies. Front Immunol. 2021, 12, 663328. [Google Scholar] [CrossRef]
- Messer, J.G.; La, S.; Kipp, D.E.; Castillo, E.J.; Yarrow, J.F.; Jorgensen, M.; Wnek, R.D.; Kimmel, D.B.; Aguirre, J.I. Diet-induced Generalized Periodontitis in Lewis Rats. Comp. Med. 2019, 69, 384–400. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Xue, Q.; He, Y. A modified method for constructing experimental rat periodontitis model. Front. Bioeng. Biotechnol. 2023, 10, 1098015. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Deng, D.; Yang, J.; Chen, Y.; Liu, J.; Zhang, M. Aggravating Effects of Psychological Stress on Ligature-Induced Periodontitis via the Involvement of Local Oxidative Damage and NF-κB Activation. Mediat. Inflamm. 2022, 2022, 6447056. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, X.; Li, W.; Wang, Q. Effects of 1,25-dihydroxyvitamin D3 on experimental periodontitis and AhR/NF-κB/NLRP3 inflammasome pathway in a mouse model. J. Appl. Oral. Sci. 2019, 27, e20180713. [Google Scholar] [CrossRef] [PubMed]
- Antonov, I.I.; Mudrov, V.P.; Nelyubin, V.N.; Muraev, A.A. Topical aspects of the chronic periodontitis immunopathogenesis (review). Clin. Dent. 2021, 1, 46–58. (In Russian) [Google Scholar] [CrossRef]
- Shamanova, Z.K.; Arutyunov, A.V.; Verevkina, Y.V.; Sirak, S.V.; Bykova, N.I.; Kobylkina, T.L. Structuralmorphological interrelation of the microcirculatory bed of the perioostus of the jaws in chronic generalized periodontitis in the experiment. Clin. Dent. 2023, 26, 44–51. (In Russian) [Google Scholar]
- Sarkisyan, N.G.; Ron, G.I.; Tuzankina, I.A.; Timchenko, A.S.; Larionov, L.P.; Bakurinskikh, A.A. Federal State Budgetary Institution of Science Institute of Immunology and Physiology of the Ural Branch of the Russian Academy of Sciences, Assignee. A Method for Obtaining a Model of Chronic Periodontitis in Rats. Russian Federation. Patent RU 2545923, 10 April 2015. (In Russian). [Google Scholar]
- Gasner, N.S.; Schure, R.S. Periodontal Disease. 10 April 2023; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aveic, S.; Craveiro, R.B.; Wolf, M.; Fischer, H. Current Trends in In Vitro Modeling to Mimic Cellular Crosstalk in Periodontal Tissue. Adv. Healthc. Mater. 2021, 10, e2001269. [Google Scholar] [CrossRef] [PubMed]
- Molon, R.; Park, C.; Jin, Q.; Sugai, J.; Cirelli, J. Characterization of ligature-induced experimental periodontitis. Microsc. Res. Tech. 2018, 81, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T.; et al. Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef]
- Kuo, P.J.; Fu, E.; Lin, C.Y.; Ku, C.T.; Chiang, C.Y.; Fu, M.M.; Fu, M.W.; Tu, H.P.; Chiu, H.C. Ameliorative effect of hesperidin on ligation-induced periodontitis in rats. J. Periodontol. 2019, 90, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Ma, Y.; Hao, Z.; Chen, S.; Fu, T.; Chen, H.; Wang, H. Oral administration of 5-hydroxytryptophan aggravated periodontitis-induced alveolar bone loss in rats. Arch. Oral. Biol. 2015, 60, 789–798. [Google Scholar] [CrossRef]
- Moiseev, D.A. Features of Prevention, Treatment, and Prognosis of Dental Pulp Pathology in Patients with Chronic Periodontitis. Tver State Medical University, Tver, Russia, 2023.
- Moiseev, D.A. Method for Modeling Periodontitis in Laboratory Animals. Russian Federation. Applicant and Patent Holder of the N.I. Pirogov Russian National Research Medical University of the Ministry of Health of the Russian Federation—No. 2023115422. Patent RU. IPC G09B 23/28 (2006.01), 13 June 2023. (In Russian). [Google Scholar]
- Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/suppdocs/feddocs/oecd/oecd_gl420.pdf (accessed on 12 June 2023).
- Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed on 12 June 2023).
- Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/suppdocs/feddocs/oecd/oecd_gl425-508.pdf (accessed on 12 June 2023).
- Makarova, M.N.; Shekunova, E.V.; Rybakova, A.V.; Makarov, V.G. Sample size of laboratory animals for experimental studies. Pharmacy 2018, 67, 3–8. (In Russian) [Google Scholar] [CrossRef]
- Likhvantsev, V.V.; Yadgarov, M.Y.; Berikashvili, L.B.; Kadantseva, K.K.; Kuzovlev, A.N. Sample size estimation. Russ. J. Anaesthesiol. Reanimatol. 2020, 6, 77–86. (In Russian) [Google Scholar] [CrossRef]
- Hanspach, J.; Nagel, A.M.; Hensel, B.; Uder, M.; Koros, L.; Laun, F.B. Sample size estimation: Current practice and considerations for original investigations in MRI technical development studies. Magn. Reson. Med. 2021, 85, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Varotto, B.L.R.; Martinez, R.C.R.; Gouveia, F.V.; Antunes, G.F.; Fabri, G.M.C.; Ballester, G.; Antequera, R.; de Siqueira, S.R.D.T.; Fonoff, E.T.; Teixeira, M.J.; et al. Increased Anxiety-Like Behavior in the Acute Phase of a Preclinical Model of Periodontal Disease. Front. Neurol. 2020, 11, 598851. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, J.N.F.M.; Fonseca, K.M.; Ferreira, B.S.P.; de Freitas Alves, B.W.; Ribeiro Júnior, H.L.; Lisboa, M.R.P.; Pereira, K.M.A.; Vale, M.L.; Gondim, D.V. Electroacupuncture Reduces Inflammation but Not Bone Loss on Periodontitis in Arthritic Rats. Inflammation 2021, 44, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, L.V.; Karpova, M.N.; Klishina NYu Kukushkin, M.L. Pain sensitivity in male Wistar rats of different age after a single injection of pentylenetetrazole in subseizure and seizure doses. Russ. J. Pain 2021, 19, 5–11. (In Russian) [Google Scholar] [CrossRef]
- Fox, J.G.; Anderson, L.C.; Loew, F.M.; Quimby, F.W. (Eds.) Laboratory Animal Medicine, 2nd ed.; Academic Press: San Diego, CA, USA, 2018. [Google Scholar]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H.; et al. Recommendations for Euthanasia of Experimental Animals: Part 2. DGXT of the European Commission. Lab Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.V.; Rybakova, A.V.; Guschin, Y.A.; Vaganova, D.S.; Koptyaeva, K.Y.; Muzhikyan, A.A. Pathomorphological diagnostics of lungs at various methods of euthanasia of laboratory animals. Lab. Anim. Sci. 2018, 3, 49–60. (In Russian) [Google Scholar] [CrossRef]
- Suttie, A.E. (Ed.) Boorman’s Pathology of the Rat: Reference and Atlas, 2nd ed.; Academic Press, an Imprint of Elsevier: London, UK; San Diego, CA, USA, 2018; 728p. [Google Scholar]
- Paudel, D.; Kuramitsu, Y.; Uehara, O.; Morikawa, T.; Yoshida, K.; Giri, S.; Islam, S.T.; Kitagawa, T.; Kondo, T.; Sasaki, K.; et al. Proteomic and microbiota analyses of the oral cavity during psychological stress. PLoS ONE 2022, 17, e0268155. [Google Scholar] [CrossRef]
- Messer, J.G.; Jiron, J.M.; Chen, H.Y.; Castillo, E.J.; Mendieta Calle, J.L.; Reinhard, M.K.; Kimmel, D.B.; Aguirre, J.I. Prevalence of Food Impaction-Induced Periodontitis in Conventionally Housed Marsh Rice Rats (Oryzomys palustris). Comp. Med. 2017, 67, 43–50. [Google Scholar]
- Yarrow, J.F.; Toklu, H.Z.; Balaez, A.; Phillips, E.G.; Otzel, D.M.; Chen, C.; Wronski, T.J.; Aguirre, J.I.; Sakarya, Y.; Tümer, N.; et al. Fructose consumption does not worsen bone deficits resulting from high-fat feeding in young male rats. Bone 2016, 85, 99–106. [Google Scholar] [CrossRef]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods. 2013, 394, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Dharmawati, I.A.; Manuaba, I.B.P.; Thahir, H.; Bakta, I.M.; Astawa, I.N.M.; Sukrama, D.M.; Yasa, I.W.P.S.; Mahadewa, T.G.B.; Kartini, N.L. Pocket measurement methods in wistar rats periodontitis induced by bacteria and the installation of silk ligature: An experimental studies. Int. J. Appl. Pharm. 2019, 11, 71–74. [Google Scholar]







| Characteristics | Control Group | Main Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Animals | Number of Dentoalveolar Segments | Number of Animals | Number of Dentoalveolar Segments | |||||
| Before | FOR 7 Days | Before | For 7 Days | Before | For 7 Days | Before | For 7 Days | |
| Gingival hyperemia | 0 | 1 | 0 | 2 | 0 | 9 | 0 | 36 |
| Gingival cyanosis | 2 | 2 | 4 | 4 | 2 | 2 | 5 | 8 |
| Gingival bleeding | 0 | 1 | 0 | 0 | 0 | 10 | 0 | 40 |
| Gingival is loose, edematous | 0 | 1 | 0 | 0 | 0 | 11 | 0 | 44 |
| Tooth mobility, up to 1 mm in the mesiodistal direction | 3 | 3 | 5 | 5 | 2 | 3 | 4 | 2 |
| Mobility of teeth, more than 1 mm in the mesiodistal direction | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 40 |
| Probing with a depth of 3–4 mm | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 23 |
| Probing with a depth of more than 4 mm | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Complex index of inflammation | 5 | 6 | 9 | 11 | 4 | 55 | 9 | 195 |
| p-value (Wilcoxon criterion) | p > 0.05 | p > 0.05 | p ≤ 0.05 | p ≤ 0.05 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiseev, D.; Donskov, S.; Dubrovin, I.; Kulyukina, M.; Vasil’ev, Y.; Volel, B.; Shadieva, S.; Babaev, A.; Shevelyuk, J.; Utyuzh, A.; et al. A New Way to Model Periodontitis in Laboratory Animals. Dent. J. 2023, 11, 219. https://doi.org/10.3390/dj11090219
Moiseev D, Donskov S, Dubrovin I, Kulyukina M, Vasil’ev Y, Volel B, Shadieva S, Babaev A, Shevelyuk J, Utyuzh A, et al. A New Way to Model Periodontitis in Laboratory Animals. Dentistry Journal. 2023; 11(9):219. https://doi.org/10.3390/dj11090219
Chicago/Turabian StyleMoiseev, Denis, Sergey Donskov, Ivan Dubrovin, Mariya Kulyukina, Yuriy Vasil’ev, Beatrice Volel, Shodiya Shadieva, Aleksey Babaev, Juliya Shevelyuk, Anatolij Utyuzh, and et al. 2023. "A New Way to Model Periodontitis in Laboratory Animals" Dentistry Journal 11, no. 9: 219. https://doi.org/10.3390/dj11090219