Highly Sensitive Ethylene Sensors Based on Ultrafine Pd Nanoparticles-Decorated Porous ZnO Nanosheets and Their Application in Fruit Ripeness Detection
Abstract
:1. Introduction
2. Experimental Method
2.1. Chemicals and Reagents
2.2. Preparation of the Porous ZnO Nanosheets
2.3. Preparation of the Ultrafine Pd Nanoparticles-Decorated ZnO Nanosheets
2.4. Measurement System of the Sensor
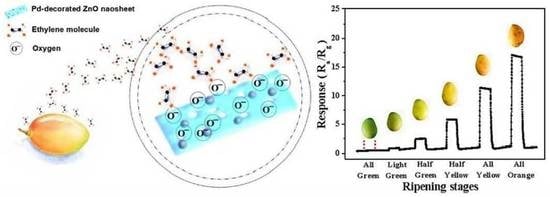
2.5. Mango Test of Ripening Stages
3. Results and Discussion
3.1. Characterization of the UPNP ZnO Nanosheets and ZnO Nanosheets
3.2. Operating Temperature of the Pure and Pd-Decorated ZnO Nanosheet-Based Sensor to Ethylene
3.3. Gas-Sensing Properties of the UPNP ZnO Nanosheets for Ethylene
3.4. Stability of the Pd-Decorated ZnO Nanosheet-Based Sensors
3.5. Sensing Mechanism of the UPNP ZnO Nanosheets
3.6. Ripeness Detection of Mangos
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fukao, T.; Bailey-Serres, J. Ethylene—A key regulator of submergence responses in rice. Plant Sci. 2008, 175, 43–51. [Google Scholar] [CrossRef]
- Grossmann, K.; Kwiatkowski, J.; Caspar, G. Regulation of Phytohormone Levels, Leaf Senescence and Transpiration by the Strobilurin Kresoxim-methyl in Wheat (Triticum aestivum). J. Plant Physiol. 1999, 154, 805–808. [Google Scholar] [CrossRef]
- Achard, P.; Cheng, H.; Grauwe, L.D. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006, 311, 124–135. [Google Scholar] [CrossRef]
- Ergun, M.; Huber, D.J. Suppression of ethylene perception extends shelf-life and quality of ‘sunrise solo’ papaya fruit at both pre-ripe and ripe stages of development. Eur. J. Hortic. Sci. 2004, 69, 184–192. [Google Scholar]
- Hanson, A.D.; Kende, H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975, 55, 663–669. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2011, 49, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Trainotti, L.; Tadiello, A.; Casadoro, G. The involvement of auxin in the ripening of climacteric fruits comes of age: The hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 2007, 58, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.; Nicometo, M.; Emond, J.P.; Melis, R.B.; Uysal, I. Improvement in fresh fruit and vegetable logistics quality: Berry logistics field studies, Philosophical transactions. Ser. A Math. Phys. Eng. Sci. 2014, 372, 201–220. [Google Scholar]
- Janssen, S.; Schmitt, K.; Blanke, M.; Bauersfeld, M.L.; Wöllenstein, J.; Lang, W. Ethylene detection in fruit supply chains. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130311. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- Woltering, E.J.; Harren, F.; Boerrigter, H.A.M. Use of a Laser-Driven Photoacoustic Detection System for Measurement of Ethylene Production in Cymbidium Flowers. Plant Physiol. 1988, 88, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Giberti, A.; Carotta, M.C.; Guidi, V.; Malagù, C.; Martinelli, G.; Piga, M.; Vendemiati, B. Monitoring of ethylene for agro-alimentary applications and compensation of humidity effects. Sens. Actuators B Chem. 2004, 103, 272–276. [Google Scholar] [CrossRef]
- Pham-Tuan, H.; Vercammen, J.; Devos, C.; Sandra, P. Automated capillary gas chromatographic system to monitor ethylene emitted from biological materials. J. Chromatogr. A 2000, 868, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Steffen, J.; Walter, L. Application of a miniaturised packed gas chromatography column and a SnO2 gas detector for analysis of low molecular weight hydrocarbons with focus on ethylene detection. Sens. Actuators B Chem. 2013, 180, 43–49. [Google Scholar]
- Mangilal, A.; Mercyma, B.; Sudhir, S.; Kody, V. SnO2 nanoparticle-based passive capacitive sensor for ethylene detection. J. Nanomater. 2012, 2012, 5. [Google Scholar]
- Nimittrakoolchai, O.-U.; Supothina, S. High-yield precipitation synthesis of tungsten oxide platelet particle and its ethylene gas-sensing characteristic. Mater. Chem. Phys. 2008, 112, 270–274. [Google Scholar] [CrossRef]
- Peng, S.; Chen, W.; Jin, L.; Xu, L.; Teng, L.; Zhou, Q. Pt-doped SnO2 nanoflower gas sensor detection characteristic for hydrocarbon gases dissolved in transformer oil. In Proceedings of the 2016 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Chengdu, China, 9–22 September 2016; 978. [Google Scholar] [CrossRef]
- Jadsadapattarakul, C.; Thanachayanont, D.; Nukeaw, C.; Sooknoi, J. Improved selectivity, response time and recovery time by [010] highly preferred-orientation silicalite-1 layer coated on SnO2 thin film sensor for selective ethylene gas detection. Sens. Actuators B Chem. 2010, 144, 73–80. [Google Scholar] [CrossRef]
- Ngam, P.; Jiemsirilers, Y.; Supothina, S. Preparation of tungsten oxide–tin oxide nanocomposites and their ethylene sensing characteristics. Sens. Actuators A Phys. 2007, 139, 7–11. [Google Scholar] [CrossRef]
- Kanungo, D.; Saha, J.; Basu, H. Pd sensitized porous silicon hydrogen sensor -Influence of ZnO thin film. Sens. Actuators B Chem. 2010, 147, 128–136. [Google Scholar] [CrossRef]
- Winquist, F.; Spetz, A.; Armgarth, M.; Nylander, C.; Lundström, I. Modified palladium metal-oxide-semiconductor structures with increased ammonia gas sensitivity. Appl. Phys. Lett. 1983, 43, 839–841. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Y.; Xu, Q.; Xu, J.Q.; Pan, P. Self-assemblies of Pd nanoparticles on the surfaces of single crystal ZnO nanowires for chemical sensors with enhanced performances. J. Mater. Chem. 2009, 19, 4701. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced Gas Sensing by Individual SnO2 Nanowires and Nanobelts Functionalized with Pd Catalyst Particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kamienchick, D.J.; Youn, I.; Rothschild, D.Y.; Kim, A. Ultrasensitive and highly selective gas sensors based on elec-trospun SnO2 nanofibers modified by Pd loading. Adv. Funct. Mater. 2010, 20, 4258–4264. [Google Scholar] [CrossRef]
- Wilson, O.M.; Knecht, M.R.; Garcia-Martinez, J.C.; Crooks, R.M. Effect of Pd Nanoparticle Size on the Catalytic Hydrogenation of Allyl Alcohol. J. Am. Chem. Soc. 2006, 128, 4510–4511. [Google Scholar] [CrossRef]
- Lee, W.Z. Particle Size Effects in Pd-Catalyzed Electrooxidation of Formic Acid. J. Phys. Chem. C 2008, 112, 3789–3793. [Google Scholar]
- Cheng, X.L.; Zhao, X.L.; Huo, H.; Gao, L.H.; Zhao, S. ZnO nanoparticulate thin film: Preparation, characterization and gas-sensing property. Sens. Actuators B Chem. 2004, 102, 248–252. [Google Scholar] [CrossRef]
- Look, D.C. Electrical and optical properties of p-type ZnO. Semicond. Sci. Technol. 2005, 20, S55–S61. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Shooshtari, M.; Pahlavan, S.; Rahbarpour, S.; Ghafoorifard, H. Investigating Organic Vapor Sensing Properties of Composite Carbon Nanotube-Zinc Oxide Nanowire. Chemosensors 2022, 10, 205. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, J.; Wang, J.; Yang, L.W. 3D hierarchically porous ZnO structures and their functionalization by aunanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. [Google Scholar] [CrossRef]
- Meng, F.; Hou, N.; Ge, S.; Sun, B.; Jin, Z.; Shen, W.; Kong, L.; Guo, Z.; Sun, Y.; Wu, H.; et al. Flower-like hierarchical structures consisting of porous single-crystalline ZnO nanosheets and their gas sensing properties to volatile organic compounds (VOCs). J. Alloy. Compd. 2014, 626, 124–130. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Baratto, C.; Comini, E.; Faglia, G.; Ferroni, M.; Ponzoni, A.; Vomiero, A. Synthesis and characterization of semiconducting nanowires for gas sensing. Sens. Actuators B Chem. 2007, 121, 208–213. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, B.; Su, Q.; Xie, E.; Lan, W. A simple method for the preparation of hollow ZnO nanospheres for use as a high per-formance photocatalyst. Nanoscale 2012, 4, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Bie, L.-J.; Yan, X.-N.; Yin, J.; Duan, Y.-Q.; Yuan, Z.-H. Nanopillar ZnO gas sensor for hydrogen and ethanol. Sens. Actuators B Chem. 2007, 126, 604–608. [Google Scholar] [CrossRef]
- Kocemba, I.; Rynkowski, J. The effect of oxygen adsorption on catalytic activity of SnO2 in CO oxidation. Catal. Today 2011, 169, 192–199. [Google Scholar] [CrossRef]
- Kohl, D. Surface processes in the detection of reducing gases with SnO2-based devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Alenezi, M.; Jayawardena, A.S.; Beliatis, K.; Henley, M.J.; Silva, S.J. Role of The Exposed Polar Facets in The Performance of Thermally and UV Activated ZnO nanostructured Gas Sensors. Sens. J. Phys. Chem. C 2013, 23, 17850–17858. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Wang, D.-C.; Xie, W.-J.; Ding, Y.; Li, J. Highly Sensitive Ethylene Sensors Based on Ultrafine Pd Nanoparticles-Decorated Porous ZnO Nanosheets and Their Application in Fruit Ripeness Detection. Processes 2023, 11, 1686. https://doi.org/10.3390/pr11061686
Jin Z, Wang D-C, Xie W-J, Ding Y, Li J. Highly Sensitive Ethylene Sensors Based on Ultrafine Pd Nanoparticles-Decorated Porous ZnO Nanosheets and Their Application in Fruit Ripeness Detection. Processes. 2023; 11(6):1686. https://doi.org/10.3390/pr11061686
Chicago/Turabian StyleJin, Zhen, De-Cai Wang, Wen-Jie Xie, Yi Ding, and Jie Li. 2023. "Highly Sensitive Ethylene Sensors Based on Ultrafine Pd Nanoparticles-Decorated Porous ZnO Nanosheets and Their Application in Fruit Ripeness Detection" Processes 11, no. 6: 1686. https://doi.org/10.3390/pr11061686