Effects of Calcium Sulfate and Chitosan on Textural Modification and Microstructure of Tofu Made from Lentils (Lens culinaris)
Abstract
:1. Introduction
2. Materials and Methods
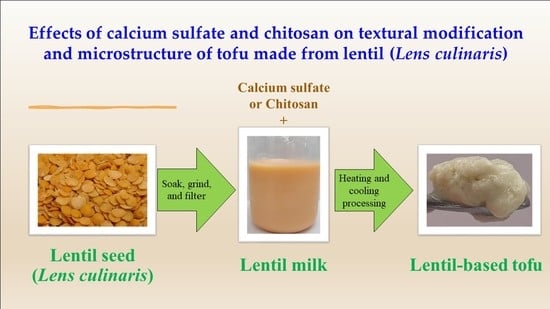
2.1. Preparation of Lentil-Based Tofu
2.2. Assessment of Gel Properties
2.3. Microstructure of Lentil-Based Tofu
2.4. Preparation of Lentil Milk Samples
2.5. SDS-PAGE Analysis of LMSF
2.6. Extraction and Analysis of Total Phenolic Content in LMPF Samples
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Calcium Sulfate and Chitosan on the Gel Properties of Lentil-Based Tofu
3.2. Effect of Calcium Sulfate and Chitosan on the Appearance of Lentil-Based Tofu
3.3. Effect of Calcium Sulfate and Chitosan on the Microstructure of Lentil-Based Tofu
3.4. SDS-PAGE Analysis of Calcium Sulfate and Chitosan on the Coagulation of Lentil Proteins in LMSF Samples
3.5. Effect of Calcium Sulfate and Chitosan on the TPC in LMPF Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarpa-Parra, M. Lentil protein: A review of functional properties and food application. An overview of lentil protein functionality. Int. J. Food Sci. Technol. 2018, 53, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.J.; Huang, W.; Chen, L. Fabrication and characterization of lentil protein gels from fibrillar aggregates and the gelling mechanism study. Food Funct. 2020, 11, 10114–10125. [Google Scholar] [CrossRef] [PubMed]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of lentil protein extraction and the influence of process pH on protein structure and functionality. LWT-Food Sci. Technol. 2014, 57, 461–469. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Yu, C.J.; Li, W.T.; Hsieh, J.F. Coagulation of β-conglycinin, glycinin and isoflavones induced by calcium chloride in soymilk. Sci. Rep. 2015, 5, 13018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, Y.H.; Hsia, S.Y.; Chan, Y.C.; Hsieh, J.F. Complex coacervation of soy proteins, isoflavones and chitosan. Molecules 2017, 22, 1022. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Li-Chan, E.C.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Kilara, A. Aggregation induced by calcium chloride and subsequent thermal gelation of whey protein isolate. J. Dairy Sci. 1998, 81, 925–931. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation of tofu using chitosan as a coagulant for improved shelf-life. Int. J. Food Sci. Technol. 2004, 39, 133–141. [Google Scholar] [CrossRef]
- Kim, M.; Son, I.S.; Han, J.S. Evaluation of microbiological, physicochemical and sensory qualities of chitosan tofu during storage. J. Food Qual. 2004, 27, 27–40. [Google Scholar] [CrossRef]
- DePalma, K.; Smith, B.; McDonald, A.G. Synergistic effects of processing parameters on the biochemical and physical properties of tofu made from yellow field pea (Pisum sativum), as determined by response surface methodology. Food Sci. Nutr. 2021, 9, 1132–1142. [Google Scholar] [CrossRef]
- Benmeziane-Derradji, F.; Djermoune-Arkoub, L.; Ayat, N.E.H.; Aoufi, D. Impact of roasting on the physicochemical, functional properties, antioxidant content and microstructure changes of Algerian lentil (Lens culinaris) flour. J. Food Meas. Charact. 2020, 14, 2840–2853. [Google Scholar] [CrossRef]
- Jao, C.H.; Kuo, M.I.; Chen, C.J.; Hsieh, J.F. Influence of chitosan and glucono-δ-lactone on the gel properties, microstructural and textural modification of pea-based tofu-type product. Processes 2022, 10, 1639. [Google Scholar] [CrossRef]
- Liu, H.H.; Chien, J.T.; Kuo, M.I. Ultra high pressure homogenized soy flour for tofu making. Food Hydrocoll. 2013, 32, 278–285. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Ge, J.; Fang, Y. Modulation of oligoguluronate on the microstructure and properties of Ca-dependent soy protein gels. Carbohyd. Polym. 2020, 250, 116920. [Google Scholar] [CrossRef]
- Hsia, S.Y.; Hsiao, Y.H.; Li, W.T.; Hsieh, J.F. Aggregation of soy protein-isoflavone complexes and gel formation induced by glucono-δ-lactone in soymilk. Sci. Rep. 2016, 6, 35718. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.C.; Hsiao, Y.H.; Hsieh, J.F. Catechin content and free radical scavenging activity of Camellia sinensis twig extracts. Int. Food Res. J. 2021, 28, 248–254. [Google Scholar] [CrossRef]
- Mhatre, S.S.; Jain, S.K.; Murdia, L.K.; Jain, H.K. Effects of different coagulation temperatures on the texture and yield of soy paneer (tofu). Int. J. Food Eng. 2008, 4, 8. [Google Scholar] [CrossRef]
- Byars, J.A.; Singh, M. Rheological and textural properties of pulse starch gels. Starch-Stärke 2016, 68, 778–784. [Google Scholar] [CrossRef]
- Kao, F.J.; Su, N.W.; Lee, M.H. Effect of calcium sulfate concentration in soymilk on the microstructure of firm tofu and the protein constitutions in tofu whey. J. Agric. Food Chem. 2003, 51, 6211–6216. [Google Scholar] [CrossRef]
- Kim, M.; Han, J.S. Evaluation of physico-chemical characteristics and microstructure of tofu containing high viscosity chitosan. Int. J. Food Sci. Technol. 2002, 37, 277–283. [Google Scholar] [CrossRef]
- Chen, K.; Huang, Y.; Li, X.; Wu, Y.; Liu, Y.; Wang, F. Textural properties of firm tofu as affected by calcium coagulants. J. Food Meas. Charact. 2021, 15, 4508–4516. [Google Scholar] [CrossRef]
- Zhao, H.; Li, W.; Qin, F.; Chen, J. Calcium sulphate-induced soya bean protein tofu-type gels: Influence of denaturation and particle size. Int. J. Food Sci. Technol. 2016, 51, 731–741. [Google Scholar] [CrossRef]
- amar Cheba, B. Chitosan: Properties, modifications and food nanobiotechnology. Procedia Manuf. 2020, 46, 652–658. [Google Scholar] [CrossRef]
- Li, X.; Xia, W. Effects of chitosan on the gel properties of salt-soluble meat proteins from silver carp. Carbohyd. Polym. 2010, 82, 958–964. [Google Scholar] [CrossRef]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chem. X 2021, 9, 100112. [Google Scholar] [CrossRef] [PubMed]
- Ono, T. Soy (soya) cheeses. In Encyclopedia of Food Science and Nutrition; Caballero, B., Trugo, L.C., Finglas, P.M., Eds.; Academic Press: London, UK, 2003; pp. 5398–5402. [Google Scholar]
- Huang, G.Q.; Sun, Y.T.; Xiao, J.X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. Phytochemicals of lentil (Lens culinaris) and their antioxidant and anti-inflammatory effects. J. Food Bioact. 2018, 1, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohyd. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef]
- Ren, J.; Li, Q.; Dong, F.; Feng, Y.; Guo, Z. Phenolic antioxidants-functionalized quaternized chitosan: Synthesis and antioxidant properties. Int. J. Biol. Macromol. 2013, 53, 77–81. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jao, C.-H.; Lin, C.-Y.; Chen, C.-J.; Hsieh, J.-F. Effects of Calcium Sulfate and Chitosan on Textural Modification and Microstructure of Tofu Made from Lentils (Lens culinaris). Processes 2022, 10, 2000. https://doi.org/10.3390/pr10102000
Jao C-H, Lin C-Y, Chen C-J, Hsieh J-F. Effects of Calcium Sulfate and Chitosan on Textural Modification and Microstructure of Tofu Made from Lentils (Lens culinaris). Processes. 2022; 10(10):2000. https://doi.org/10.3390/pr10102000
Chicago/Turabian StyleJao, Cheng-Hsun, Chieh-Yi Lin, Chao-Jung Chen, and Jung-Feng Hsieh. 2022. "Effects of Calcium Sulfate and Chitosan on Textural Modification and Microstructure of Tofu Made from Lentils (Lens culinaris)" Processes 10, no. 10: 2000. https://doi.org/10.3390/pr10102000