Separation of Free Fatty Acid and Triglycerides by Molecular Distillation–Experimental and Simulation Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and FFA Separation
2.2. FFA Content Determination
2.3. Statistical Analysis
3. Experimental Results
3.1. Mass Ratio and FFA Content
3.2. Effect of Vapor Pressure
3.3. Effects of FFA Content and Temperature on FFA Removal from Triglycerides Mixture
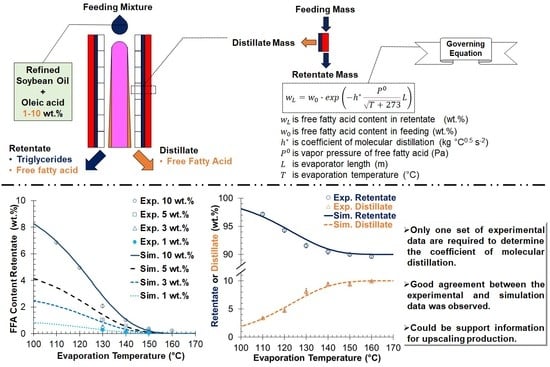
4. Modeling Discussion
4.1. Mathematical Model Approach
4.2. Comparison between the Experimental and Simulation Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture. Oilseeds: World Markets and Trade; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2022; Available online: https://usda.library.cornell.edu/concern/publications/tx31qh68h?locale=en (accessed on 14 February 2022).
- Pizzo, J.S.; Cruz, V.H.M.; Santos, P.D.S.; Silva, G.R.; Souza, P.M.; Manin, L.P.; Santos, O.O.; Visentainer, J.V. Instantaneous Characterization of Crude Vegetable Oils via Triacylglycerols Fingerprint by Atmospheric Solids Analysis Probe Tandem Mass Spectrometry with Multiple Neutral Loss Scans. Food Control 2022, 134, 108710. [Google Scholar] [CrossRef]
- Goh, E.M.; Timms, R.E. Determination of Mono- and Diglycerides in Palm Oil, Olein and Stearin. J. Am. Oil Chem. Soc. 1985, 62, 730–734. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Lipids. In Food Chemistry, 3rd ed.; Burghagen, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 157–242. [Google Scholar]
- Frega, N.; Mozzon, M.; Lercker, G. Effects of Free Fatty Acids on Oxidative Stability of Vegetable Oil. J. Am. Oil Chem. Soc. 1999, 76, 325–329. [Google Scholar] [CrossRef]
- Vaisali, C.; Charanyaa, S.; Belur, P.D.; Regupathi, I. Refining of Edible Oils: A Critical Appraisal of Current and Potential Technologies. Int. J. Food Sci. Technol. 2015, 50, 13–23. [Google Scholar] [CrossRef]
- Codex Alimentarius: International Food Standards. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 11 September 2022).
- Tarhan, İ.; Ismail, A.A.; Kara, H. Quantitative Determination of Free Fatty Acids in Extra Virgin Olive Oils by Multivariate Methods and Fourier Transform Infrared Spectroscopy Considering Different Absorption Modes. Int. J. Food Prop. 2017, 20 (Suppl. S1), S790–S797. [Google Scholar] [CrossRef] [Green Version]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Edible Fat and Oils. In Food Chemistry, 3rd ed.; Burghagen, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 643–671. [Google Scholar]
- Gibon, V.; De Greyt, W.; Kellens, M. Palm Oil Refining. Eur. J. Lipid Sci. Technol. 2007, 109, 315–335. [Google Scholar] [CrossRef]
- Franke, K.; Strijowski, U.; Fleck, G.; Pudel, F. Influence of Chemical Refining Process and Oil Type on Bound 3-Chloro-1,2-Propanediol Contents in Palm Oil and Rapeseed Oil. LWT-Food Sci. Technol. 2009, 42, 1751–1754. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef]
- Čmolík, J.; Pokorný, J. Physical Refining of Edible Oils. Eur. J. Lipid Sci. Technol. 2000, 102, 472–486. [Google Scholar] [CrossRef]
- Forster, A.; Harper, A.J. Physical Refining. J. Am. Oil Chem. Soc. 1983, 60, 265–271. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Tekin, A. Applications of molecular distillation technique in food products. Ital. J. Food Sci. 2015, 27, 277–281. [Google Scholar]
- Biehler, R.M.; Hickman, K.C.D.; Perry, E.S. Small Laboratory Centrifugal Molecular Still. Anal. Chem. 1949, 21, 638–640. [Google Scholar] [CrossRef]
- Lutisan, J.; Cvengros, J. Mean Free Path of Molecules on Molecular Distillation. J. Chem. Eng. 1995, 56, 39–50. [Google Scholar] [CrossRef]
- Batistella, C.B.; Wolf Maciel, M.R. Recovery of Carotenoids from Palm Oil by Molecular Distillation. Comput. Chem. Eng. 1998, 22, S53–S60. [Google Scholar] [CrossRef]
- Cvengros, J. Physical Refining of Edible Oils. J. Am. Oil Chem. Soc. 1995, 72, 1193–1196. [Google Scholar] [CrossRef]
- Cermak, S.C.; John, A.L.; Evangelista, R.L. Enrichment of Decanoic Acid in Cuphea Fatty Acids by Molecular Distillation. Ind. Crops Prod. 2007, 26, 93–99. [Google Scholar] [CrossRef]
- Dantas, T.N.C.; Cabral, T.J.O.; Dantas Neto, A.A.; Moura, M.C.P.A. Enrichmnent of Patchoulol Extracted from Patchouli (Pogostemon Cablin) Oil by Molecular Distillation Using Response Surface and Artificial Neural Network Models. J. Ind. Eng. Chem. 2020, 81, 219–227. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.-L. DSMC Simulation of Vapor Flow in Molecular Distillation. Vacuum 2014, 110, 40–46. [Google Scholar] [CrossRef]
- Tehlah, N.; Kaewpradit, P.; Mujtaba, I.M. Development of Molecular Distillation Based Simulation and Optimization of Refined Palm Oil Process Based on Response Surface Methodology. Processes 2017, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- AOAC International. AOAC Official Method 940.28; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care 2004, 8, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.; Wuensch, K.L. SPSS and SAS Programs for Comparing Pearson Correlations and OLS Regression Coefficients. Behav. Res. 2013, 45, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.F.; Ito, V.M.; Batistella, C.B.; Maciel, M.R.W. Free Fatty Acid Separation from Vegetable Oil Deodorizer Distillate Using Molecular Distillation Process. Sep. Purif. Technol. 2006, 48, 78–84. [Google Scholar] [CrossRef]
- Tan, C.-H.; Ghazali, H.M.; Kuntom, A.; Tan, C.-P.; Ariffin, A.A. Extraction and Physicochemical Properties of Low Free Fatty Acid Crude Palm Oil. Food Chem. 2009, 113, 645–650. [Google Scholar] [CrossRef]
- Thomson, G.W. The Antoine Equation for Vapor-Pressure Data. Chem. Rev. 1946, 38, 1–39. [Google Scholar] [CrossRef]
- Matricarde Falleiro, R.M.; Akisawa Silva, L.Y.; Meirelles, A.J.A.; Krähenbühl, M.A. Vapor Pressure Data for Fatty Acids Obtained Using an Adaptation of the DSC Technique. Thermochim. Acta 2012, 547, 6–12. [Google Scholar] [CrossRef]
- Zong, L.; Ramanathan, S.; Chen, C.-C. Predicting Thermophysical Properties of Mono- and Diglycerides with the Chemical Constituent Fragment Approach. Ind. Eng. Chem. Res. 2010, 49, 5479–5484. [Google Scholar] [CrossRef]
- Wada, S.; Koizumi, C.; Nonaka, J. Analysis of Triglycerides of Soybean Oil by High-Performance Liquid Chromatography in Combination with Gas Liquid Chromatography. J. Jpn. Oil Chem. Soc. 1977, 26, 95–99. [Google Scholar] [CrossRef]
- Himmelblau, D.M.; Riggs, J.B. Multiphase Equilibrium. In Basic Principles and Calculation in Chemical Engineering, 8th ed.; Pearson Education: Bergen, NJ, USA, 2012; pp. 411–466. [Google Scholar]
- Wang, Y.; Zhao, M.; Song, K.; Wang, L.; Han, X.; Tang, S.; Wang, Y. Separation of Diacylglycerols from Enzymatically Hydrolyzed Soybean Oil by Molecular Distillation. Sep. Purif. Technol. 2010, 75, 114–120. [Google Scholar] [CrossRef]
- Saliu, F.; Longhin, E.; Salanti, A.; Degano, I.; Della Pergola, R. Sphingoid Esters from the Molecular Distillation of Squid Oil: A Preliminary Bioactivity Determination. Food Chem. 2016, 201, 23–28. [Google Scholar] [CrossRef]
- Rodriguez, N.E.; Martinello, M.A. Molecular Distillation Applied to the Purification of Biodiesel from Ethanol and Soybean Oil. Fuel 2021, 296, 120597. [Google Scholar] [CrossRef]
- Holland, L.; Steckelmacher, W.; Yarwood, J. Basic data. In Vacuum Manual, 1st ed.; E. & F. N. Spon: London, UK, 1974; pp. 1–83. [Google Scholar]
- Ştefan, N.G.; Iancu, P.; Pleșu, V.; Călinescu, I.; Ignat, N.D. Highly Efficient Deacidification Process for Camelina Sativa Crude Oil by Molecular Distillation. Sustainability 2021, 13, 2818. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Sahin Ozkan, K.; Yorulmaz, A.; Tekin, A. Molecular Distillation of Olive Pomace Oil—Multiobjective Optimization for Tocopherol and Squalene. LWT 2018, 91, 198–202. [Google Scholar] [CrossRef]
- Shimada, Y.; Nakai, S.; Suenaga, M.; Sugihara, A.; Kitano, M.; Tominaga, Y. Facile Purification of Tocopherols from Soybean Oil Deodorizer Distillate in High Yield Using Lipase. J. Am. Oil Chem. Soc. 2000, 77, 1009–1013. [Google Scholar] [CrossRef]
- Martins, P.F.; Batistella, C.B.; Maciel-Filho, R.; Wolf-Maciel, M.R. Comparison of Two Different Strategies for Tocopherols Enrichment Using a Molecular Distillation Process. Ind. Eng. Chem. Res. 2006, 45, 753–758. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changwatchai, T.; Nakajima, M.; Felipe, L.d.O.; Neves, M.A. Separation of Free Fatty Acid and Triglycerides by Molecular Distillation–Experimental and Simulation Approaches. Processes 2022, 10, 2053. https://doi.org/10.3390/pr10102053
Changwatchai T, Nakajima M, Felipe LdO, Neves MA. Separation of Free Fatty Acid and Triglycerides by Molecular Distillation–Experimental and Simulation Approaches. Processes. 2022; 10(10):2053. https://doi.org/10.3390/pr10102053
Chicago/Turabian StyleChangwatchai, Teetach, Mitsutoshi Nakajima, Lorena de Oliveira Felipe, and Marcos A. Neves. 2022. "Separation of Free Fatty Acid and Triglycerides by Molecular Distillation–Experimental and Simulation Approaches" Processes 10, no. 10: 2053. https://doi.org/10.3390/pr10102053