Assessment of Cardiovascular Function in Childhood Leukemia Survivors: The Role of the Right Heart
Abstract
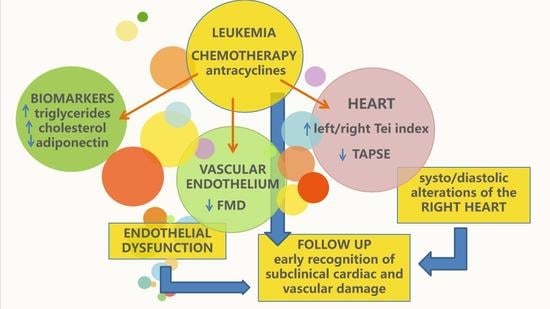
:1. Introduction
2. Methods
2.1. Study Population
2.2. Auxological Parameters
2.3. Biochemical and Hemostatic Markers
2.4. Cardiovascular US Measurement
2.5. Evaluation of Mean-IMT
2.6. Evaluation of FMD
2.7. Echocardiography and TDI
2.8. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mulrooney, D.A.; Hyun, G.; Ness, K.K.; Bhakta, N.; Pui, C.H.; Ehrhardt, M.J.; Krull, K.R.; Crom, D.B.; Chemaitilly, W.; Srivastava, D.K.; et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: A retrospective analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019, 6, e306–e316. [Google Scholar] [CrossRef]
- Faienza, M.F.; Delvecchio, M.; Giordano, P.; Cavallo, L.; Grano, M.; Brunetti, G.; Ventura, A. Metabolic syndrome in childhood leukemia survivors: A meta-analysis. Endocrine 2015, 49, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial dysfunction and cardiovascular risk factors in childhood acute lymphoblastic leukemia survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; Muggeo, P.; Monteduro, M.; Lassandro, G.; Novielli, C.; Valente, F.; Salinaro, E.; Zito, A.; Ciccone, M.M.; Miniello, V.L.; et al. Non-alcoholic fatty liver disease is associated with early left ventricular dysfunction in childhood acute lymphoblastic leukaemia survivors. Eur. J. Endocrinol. 2017, 176, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Muggeo, P.; Muggeo, V.M.R.; Giordano, P.; Delvecchio, M.; Altomare, M.; Novielli, C.; Ciccone, M.M.; D’Amato, G.; Faienza, M.F.; Santoro, N. Cardiovascular dysfunction and vitamin D status in childhood acute lymphoblastic leukemia survivors. World J. Pediatr. 2019, 15, 465–470. [Google Scholar] [CrossRef]
- Lipshultz, E.R.; Chow, E.J.; Doody, D.R.; Armenian, S.H.; Asselin, B.L.; Baker, K.S.; Bhatia, S.; Constine, L.S.; Freyer, D.R.; Kopp, L.M.; et al. Cardiometabolic Risk in Childhood Cancer Survivors: A Report from the Children’s Oncology Group. Cancer Epidemiol. Biomark. Prev. 2022, 31, 536–542. [Google Scholar] [CrossRef]
- Pluimakers, V.G.; van Waas, M.; Neggers, S.J.C.M.M.; van den Heuvel-Eibrink, M.M. Metabolic syndrome as cardiovascular risk factor in childhood cancer survivors. Crit. Rev. Oncol. Hematol. 2019, 133, 129–141. [Google Scholar] [CrossRef]
- Shimomura, Y.; Baba, R.; Watanabe, A.; Horikoshi, Y.; Asami, K.; Hyakuna, N.; Iwai, A.; Matsushita, T.; Yamaji, K.; Hori, T.; et al. Japanese Childhood Cancer and Leukemia Study Group (JCCLSG). Assessment of late cardiotoxicity of pirarubicin (THP) in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2011, 57, 461–466. [Google Scholar] [CrossRef]
- Rajapreyar, P.; Lorenzana, A.; Prabhu, A.; Szpunar, S.; Anne, P. Tissue Doppler Imaging and Focal, Late-Onset Anthracycline-Induced Cardiovascular Disease in Long Term Survivors of Childhood Cancer: A Research Article. J. Clin. Diagn. Res. 2016, 10, SC01–SC04. [Google Scholar] [CrossRef]
- Samosir, S.M.; Utamayasa, I.K.A.; Andarsini, M.R.; Rahman, M.A.; Ontoseno, T.; Hidayat, T.; Ugrasena, I.D.G.; Larasati, M.C.S.; Cahyadi, A. Risk Factors of Daunorubicine Induced Early Cardiotoxicity in Childhood Acute Lymphoblastic Leukemia: A Retrospective Study. Asian Pac. J. Cancer Prev. 2021, 22, 1407–1412. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallarossa, P.; Maurea, N.; Cadeddu, C.; Madonna, R.; Mele, D.; Monte, I.; Novo, G.; Pagliaro, P.; Pepe, A.; Tocchetti, C.G.; et al. A recommended practical approach to the management of anthracycline-based chemotherapy cardiotoxicity: An opinion paper of the working group on drug cardiotoxicity and cardioprotection, Italian Society of Cardiology. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), e84–e92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seara, F.A.C.; Kasai-Brunswick, T.H.; Nascimento, J.H.M.; Campos-de-Carvalho, A.C. Anthracycline-induced cardiotoxicity and cell senescence: New therapeutic option? Cell Mol. Life Sci. 2022, 79, 568. [Google Scholar] [CrossRef]
- Vandecruys, E.; Mondelaers, V.; De Wolf, D.; Benoit, Y.; Suys, B. Late cardiotoxicity after low dose of anthracycline therapy for acute lymphoblastic leukemia in childhood. J. Cancer Surviv. 2012, 6, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Amedro, P.; Vincenti, M.; Abassi, H.; Lanot, N.; De La Villeon, G.; Guillaumont, S.; Gamon, L.; Mura, T.; Lopez-Perrin, K.; Haouy, S.; et al. Use of speckle tracking echocardiography to detect late anthracycline-induced cardiotoxicity in childhood cancer: A prospective controlled cross-sectional study. Int. J. Cardiol. 2022, 354, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.R.; Massey, R.; Dalen, H.; Kanellopoulos, A.; Hamre, H.; Ruud, E.; Kiserud, C.E.; Fosså, S.D.; Aakhus, S. Right ventricular function in long-term adult survivors of childhood lymphoma and acute lymphoblastic leukaemia. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Bergler-Klein, J. Right from the heart: Survivors of childhood cancer and the right ventricle. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 742–743. [Google Scholar] [CrossRef] [Green Version]
- Lenčová-Popelová, O.; Jirkovský, E.; Mazurová, Y.; Lenčo, J.; Adamcová, M.; Šimůnek, T.; Geršl, V.; Štěrba, M. Molecular remodeling of left and right ventricular myocardium in chronic anthracycline cardiotoxicity and post-treatment follow up. PLoS ONE 2014, 9, e96055. [Google Scholar] [CrossRef]
- Jenei, Z.; Bárdi, E.; Magyar, M.T.; Horváth, A.; Paragh, G.; Kiss, C. Anthracycline causes impaired vascular endothelial function and aortic stiffness in long term survivors of childhood cancer. Pathol. Oncol. Res. 2013, 19, 375–383. [Google Scholar] [CrossRef]
- Sadurska, E.; Zaucha-Prażmo, A.; Brodzisz, A.; Kowalczyk, J.; Beń-Skowronek, I. Premature atherosclerosis after treatment for acute lymphoblastic leukemia in childhood. Ann. Agric. Environ. Med. 2018, 25, 71–76. [Google Scholar] [CrossRef]
- Parr, S.K.; Liang, J.; Schadler, K.L.; Gilchrist, S.C.; Steele, C.C.; Ade, C.J. Anticancer Therapy-Related Increases in Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e015598. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Mammadova, A.; Benkirane-Jessel, N.; Désaubry, L.; Nebigil, C.G. Updates on Anticancer Therapy-Mediated Vascular Toxicity and New Horizons in Therapeutic Strategies. Front. Cardiovasc. Med. 2021, 8, 694711. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Flegal, K.M.; Carroll, M.D.; Johnson, C.L. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 2002, 288, 1728–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutfield, W.S.; Jefferies, C.A.; Jackson, W.E.; Robinson, E.M.; Hofman, P.L. Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr. Diabetes 2003, 4, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Delvecchio, M.; Zito, A.; De Palma, F.; Cortese, F.; Nitti, A.; Massari, E.; Gesualdo, M.; Ricci, G.; et al. Vascular Function and Myocardial Performance Indices in Children Born Small for Gestational Age. Circ. J. 2016, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Pignoli, P.; Tremoli, E.; Poli, A.; Oreste, P.; Paoletti, R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation 1986, 74, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; Wiley: New York, NY, USA, 1986; pp. 5–12. [Google Scholar]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Ciccone, M.M.; Miniello, V.; Marchioli, R.; Scicchitano, P.; Cortese, F.; Palumbo, V.; Primitivo, S.G.; Sassara, M.; Ricci, G.; Carbonara, S.; et al. Morphological and functional vascular changes induced by childhood obesity. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 831–835. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Favale, S.; Bhuva, A.; Scicchitano, P.; Caragnano, V.; Lavopa, C.; De Pergola, G.; Loverro, G. Anteroposterior diameter of the infrarenal abdominal aorta is higher in women with polycystic ovary syndrome. Vasc. Health Risk Manag. 2009, 5, 561–566. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Iacoviello, M.; Puzzovivo, A.; Scicchitano, P.; Monitillo, F.; De Crescenzo, F.; Caragnano, V.; Sassara, M.; Quistelli, G.; Guida, P.; et al. Clinical correlates of endothelial function in chronic heart failure. Clin. Res. Cardiol. 2011, 100, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Lenihan, D.J. Clinical Practice Guidelines in Cardio-Oncology. Heart. Fail. Clin. 2022, 18, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Tei, C. New non-invasive index for combined systolic and diastolic ventricular function. J. Cardiol. 1995, 26, 396–404. [Google Scholar]
- Franco, V.I.; Lipshultz, S.E. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol. Young 2015, 25, 107–116. [Google Scholar] [CrossRef]
- Cilluffo, G.; Sottile, G.; La Grutta, S.; Muggeo, V. The Induced Smoothed lasso: A practical framework for hypothesis testing in high dimensional regression. Stat. Methods Med. Res. 2020, 29, 765–777. [Google Scholar] [CrossRef]
- Larose, E.; Ganz, P.; Reynolds, H.G.; Dorbala, S.; Di Carli, M.F.; Brown, K.A.; Kwong, R.Y. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Motoki, H.; Borowski, A.G.; Shrestha, K.; Hu, B.; Kusunose, K.; Troughton, R.W.; Tang, W.H.; Klein, A.L. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J. Am. Soc. Echocardiogr. 2014, 27, 726–732. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R.T. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 9, H1373–H1381. [Google Scholar] [CrossRef]
- Guendouz, S.; Rappeneau, S.; Nahum, J.; Dubois-Randé, J.L.; Gueret, P.; Monin, J.L.; Lim, P.; Adnot, S.; Hittinger, L.; Damy, T. Prognostic significance and normal values of 2D strain to assess right ventricular systolic function in chronic heart failure. Circ. J. 2012, 76, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, W.R.; Santos, A.B.S.; Foppa, M.; Scolari, F.L.; Barros, F.C.; Stein, R.; da Silveira, A.D. Prognostic value of right ventricular strain and peak oxygen consumption in heart failure with reduced ejection fraction. Int. J. Cardiovasc. Imaging 2022. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Alkassas, A.; Alaarag, A. Tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure ratio as a predictor of in-hospital mortality for acute heart failure. BMC Cardiovasc. Disord. 2022, 22, 414. [Google Scholar] [CrossRef] [PubMed]
- Bayram, C.; Çetin, İ.; Tavil, B.; Yarali, N.; Ekici, F.; Isık, P.; Tunc, B. Evaluation of cardiotoxicity by tissue Doppler imaging in childhood leukemia survivors treated with low-dose anthracycline. Pediatr. Cardiol. 2015, 36, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Sadurska, E. Current Views on Anthracycline Cardiotoxicity in Childhood Cancer Survivors. Pediatr. Cardiol. 2015, 36, 1112–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çetin, S.; Babaoğlu, K.; Başar, E.Z.; Deveci, M.; Çorapçıoğlu, F. Subclinical anthracycline-induced cardiotoxicity in long-term follow-up of asymptomatic childhood cancer survivors: Assessment by speckle tracking echocardiography. Echocardiography 2018, 35, 234–240. [Google Scholar] [CrossRef]
- Kocabaş, A.; Kardelen, F.; Ertuğ, H.; Aldemir-Kocabaş, B.; Tosun, Ö.; Yeşilipek, A.; Hazar, V.; Akçurin, G. Assessment of early-onset chronic progressive anthracycline cardiotoxicity in children: Different response patterns of right and left ventricles. Pediatr. Cardiol. 2014, 35, 82–88. [Google Scholar] [CrossRef]
- Kaneko, S.; Tham, E.B.; Haykowsky, M.J.; Spavor, M.; Khoo, N.S.; Mackie, A.S.; Smallhorn, J.F.; Thompson, R.B.; Nelson, M.D. Impaired Left Ventricular Reserve in Childhood Cancer Survivors Treated With Anthracycline Therapy. Pediatr. Blood Cancer 2016, 63, 1086–1090. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Hering, D.; Venneri, L.; Danylenko, O. The influence of chemotherapy on the right ventricle: Did we forget something? Clin. Cardiol. 2017, 40, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Li, V.W.Y.; Liu, A.P.Y.; Wong, W.H.S.; Ho, K.K.H.; Yau, J.P.W.; Cheuk, D.K.L.; Cheung, Y.F. Left and Right Ventricular Systolic and Diastolic Functional Reserves Are Impaired in Anthracycline-Treated Long-Term Survivors of Childhood Cancers. J. Am. Soc. Echocardiogr. 2019, 32, 277–285. [Google Scholar] [CrossRef]
- Yildirim, A.; Tunaoğlu, F.S.; Pinarli, F.G.; Ilhan, M.; Oğuz, A.; Karadeniz, C.; Olguntürk, R.; Oğuz, D.; Kula, S. Tissue and flow myocardial performance index measurements taken during dobutamine stress echocardiography for early diagnosis of late anthracycline cardiotoxicity. Pediatr. Cardiol. 2010, 31, 96–105. [Google Scholar] [CrossRef]
- Cameli, M.; Righini, F.M.; Lisi, M.; Bennati, E.; Navarri, R.; Lunghetti, S.; Padeletti, M.; Cameli, P.; Tsioulpas, C.; Bernazzali, S.; et al. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am. J. Cardiol. 2013, 112, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Elbl, L.; Hrstkova, H.; Chaloupka, V. The late consequences of anthracycline treatment on left ventricular function after treatment for childhood cancer. Eur. J. Pediatr. 2003, 162, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; De Rosa, G.; Rizzo, D.; Leo, A.; Maurizi, P.; De Nisco, A.; Vendittelli, F.; Zuppi, C.; Mordente, A.; Riccardi, R. Myocardial performance index and biochemical markers for early detection of doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukaemia. Int. J. Clin. Oncol. 2013, 18, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.Y.; Chin, C.; Dahl, G.; Rosenthal, D.N. Anthracyclines cause endothelial injury in pediatric cancer patients: A pilot study. J. Clin. Oncol. 2006, 24, 925–928. [Google Scholar] [CrossRef]
- Brouwer, C.A.; Postma, A.; Hooimeijer, H.L.; Smit, A.J.; Vonk, J.M.; van Roon, A.M.; van den Berg, M.P.; Dolsma, W.V.; Lefrandt, J.D.; Bink-Boelkens, M.T.; et al. Endothelial damage in long-term survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3906–3913. [Google Scholar] [CrossRef] [Green Version]
- Hader, S.N.; Zinkevich, N.; Norwood Toro, L.E.; Kriegel, A.J.; Kong, A.; Freed, J.K.; Gutterman, D.D.; Beyer, A.M. Detrimental effects of chemotherapy on human coronary microvascular function. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H705–H710. [Google Scholar] [CrossRef] [PubMed]
- Dengel, D.R.; Ness, K.K.; Glasser, S.P.; Williamson, E.B.; Baker, K.S.; Gurney, J.G. Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2008, 30, 20–25. [Google Scholar] [CrossRef]
- Long, T.M.; Lee, F.; Lam, K.; Wallman, K.E.; Walwyn, T.S.; Choong, C.S.; Naylor, L.H. Cardiovascular Testing Detects Underlying Dysfunction in Childhood Leukemia Survivors. Med. Sci. Sports Exerc. 2020, 52, 525–534. [Google Scholar] [CrossRef]
- Morel, S.; Léveillé, P.; Samoilenko, M.; Franco, A.; England, J.; Malaquin, N.; Tu, V.; Cardin, G.B.; Drouin, S.; Rodier, F.; et al. Biomarkers of cardiometabolic complications in survivors of childhood acute lymphoblastic leukemia. Sci. Rep. 2020, 10, 21507. [Google Scholar] [CrossRef] [PubMed]
- Bruzzi, P.; Bigi, E.; Felici, F.; Lami, F.; Cano Garcinuno, M.D.C.; Giovanni, P.; Cellini, M.; Predieri, B.; Iughetti, L. Markers of Inflammation and Endothelial Dysfunction in Young Survivors from Acute Lymphoblastic Leukemia. J. Adolesc. Young Adult Oncol. 2021, 10, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Miniello, V.L.; Faienza, M.F.; Scicchitano, P.; Cortese, F.; Gesualdo, M.; Zito, A.; Basile, M.; Recchia, P.; Leogrande, D.; Viola, D.; et al. Insulin resistance and endothelial function in children and adolescents. Int. J. Cardiol. 2014, 174, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Hu, W.; Wang, M.; Xiao, Y. The role of the adipocytokines vaspin and visfatin in vascular endothelial function and insulin resistance in obes; children. BMC Endocr. Disord. 2019, 19, 127. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | ALL Patients (n = 54) | Controls (n = 37) | p Value |
|---|---|---|---|
| Age (years) | 9.9 ± 4.2 | 10.3 ± 2.8 | 0.52 |
| BMI (Kg/m2) | 21.1 ± 4.8 | 17.5 ± 1.9 | <0.0001 |
| Waist circumference (cm) | 71.5 ± 15.3 | 55.1 ± 7.9 | <0.0001 * |
| SBP (mmHg) | 105.6 ± 10.3 | 104.8 ± 5.7 | 0.63 * |
| DBP (mmHg) | 66.0 ± 7.6 | 63.8 ± 5.7 | 0.11 |
| Fasting glucose level (mg/dL) | 82.3 ± 6.4 | 77.4 ± 6.2 | <0.0001 * |
| Insulin (uIU/mL) | 12.5 ± 8.6 | 13.0 ± 2.6 | 0.70 |
| HOMA-IR | 2.6 ± 2.0 | 2.5 ± 0.6 | 0.79 |
| Total Cholesterol (mg/dL) | 150.7 ± 23.7 | 126.4 ± 13.0 | <0.0001 |
| LDL-C (mg/dL) | 86.4 ± 18.0 | 59.8 ± 14.1 | <0.0001 |
| HDL-C (mg/dL) | 50.6 ± 9.9 | 58.9 ± 8.2 | <0.0001 * |
| Triglycerides (mg/dL) | 68.3 ± 30.6 | 48.2 ± 15.3 | <0.0001 |
| Endothelin-1 (pg/mL) | 2.1 ± 0.6 | 2.0 ± 0.5 | 0.69 |
| Adiponectin (μg/mL) | 7.8 ± 3.0 | 9.0 ± 2.0 | 0.0288 |
| HMW Adiponectin (μg/mL) | 4.4 ± 2.4 | 5.8 ± 1.8 | 0.0059 |
| Characteristic | ALL Patients (n = 54) | Controls (n = 37) | p Value |
|---|---|---|---|
| FMD (%) | 8.7 ± 3.5 | 11.6 ± 5.0 | 0.0041 |
| Mean IMT (mm) | 0.46 ± 0.07 | 0.45 ± 0.06 | 0.22 |
| APAO (mm) | 10.1 ± 2.0 | 10.2 ± 1.9 | 0.71 |
| LVEF (%) | 59.7 ± 8.6 | 61.7 ± 6.3 | 0.22 |
| Left TEI index | 0.42 ± 0.07 | 0.36 ± 0.11 | 0.0057 |
| Right TEI index | 0.43 ± 0.10 | 0.34 ± 0.14 | 0.0021 |
| Left E/A ratio | 2.2 ± 0.7 | 2.0 ± 0.5 | 0.15 |
| Right E/A ratio | 1.9 ± 0.6 | 1.9 ± 0.6 | 0.97 |
| TAPSE (mm) | 16.9 ± 1.2 | 24.5 ± 3.7 | <0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muggeo, P.; Scicchitano, P.; Muggeo, V.M.R.; Novielli, C.; Giordano, P.; Ciccone, M.M.; Faienza, M.F.; Santoro, N. Assessment of Cardiovascular Function in Childhood Leukemia Survivors: The Role of the Right Heart. Children 2022, 9, 1731. https://doi.org/10.3390/children9111731
Muggeo P, Scicchitano P, Muggeo VMR, Novielli C, Giordano P, Ciccone MM, Faienza MF, Santoro N. Assessment of Cardiovascular Function in Childhood Leukemia Survivors: The Role of the Right Heart. Children. 2022; 9(11):1731. https://doi.org/10.3390/children9111731
Chicago/Turabian StyleMuggeo, Paola, Pietro Scicchitano, Vito Michele Rosario Muggeo, Chiara Novielli, Paola Giordano, Marco Matteo Ciccone, Maria Felicia Faienza, and Nicola Santoro. 2022. "Assessment of Cardiovascular Function in Childhood Leukemia Survivors: The Role of the Right Heart" Children 9, no. 11: 1731. https://doi.org/10.3390/children9111731