Pediatric Hypertension: A Condition That Matters
Abstract
:1. Introduction
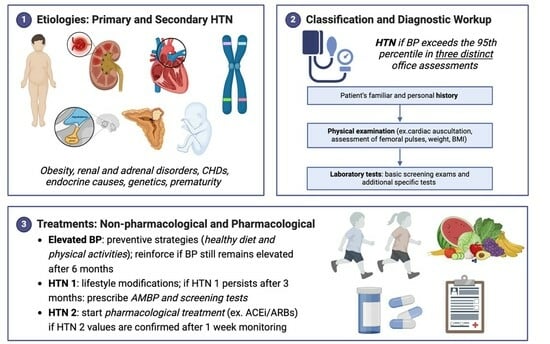
2. Etiologies
2.1. Primary Hypertension
2.2. Secondary Hypertension
3. Definition and Classification
4. Screening for HTN
5. Diagnostic Workup
- Cardiac assessment: although it has been extensively used to screen for HTN, electrocardiography is not currently recommended to rule out left ventricular hypertrophy (LVH) because of its very low positive predictive value and scarce sensitivity [50,51]. By contrast, echocardiography is the main diagnostic technique both to diagnose COA and to measure left ventricular mass, and, as consequence, potential LVH.
- 2.
- Renovascular evaluation: Doppler renal scans could be performed in children with abnormal urinalysis, renal function, or with HTN and hypokalemia to identify renal artery stenoses, especially in cooperative children aged >8 years and non-obese subjects [53]. Computed tomographic or magnetic resonance angiography may also be performed as an alternative in selected cases. The use of microalbuminuria in children as a marker of kidney damage is less established and is not currently recommended.
- 3.
- In children exhibiting low renin hypertension, hypokalemia, and with a family background of severe hypertension diagnosed during youth, refractory hypertension, cerebral vascular accidents, and heart failure causing death, genetic tests should be considered. Indeed, suspected monogenic forms such as Liddle’s syndrome, glucocorticoid-remediable aldosteronism, apparent mineralocorticoid excess, Gordon’s syndrome, mineralocorticoid receptor hypersensitivity syndrome, and hypertensive forms of congenital adrenal hyperplasia necessitate genetic analyses for accurate diagnosis in such cases [54].
6. Treatments of HTN
- Non-pharmacological treatments: when and how to start.
- -
- -
- -
- The application of techniques such as awareness meditation and yoga for stress reduction [57].
- 2.
- Pharmacologic Treatment
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassareo, P.P.; Calcaterra, G.; Sabatino, J.; Oreto, L.; Ciliberti, P.; Perrone, M.; Martino, F.; D’Alto, M.; Chessa, M.; DISalvo, G.; et al. Primary and secondary paediatric hypertension. J. Cardiovasc. Med. 2023, 24 (Suppl. S1), e77–e85. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. Pediatrics 2018, 142, e20181739. [Google Scholar] [CrossRef] [PubMed]
- Downie, M.L.; Ulrich, E.H.; Noone, D.G. An Update on Hypertension in Children With Type 1 Diabetes. Can. J. Diabetes 2018, 42, 199–204. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J. Atrial fibrillation: Hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am. J. Cardiol. 2003, 91, 9G–14G. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Malatino, L. Stroke and Hypertension: An Appraisal from Pathophysiology to Clinical Practice. Curr. Vasc. Pharmacol. 2019, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Alderman, M.H. Characteristics of children with primary hypertension seen at a referral center. Pediatr. Nephrol. 2005, 20, 961–966. [Google Scholar] [CrossRef]
- Baracco, R.; Kapur, G.; Mattoo, T.; Jain, A.; Valentini, R.; Ahmed, M.; Thomas, R. Prediction of primary vs. secondary hypertension in children. J. Clin. Hypertens. 2012, 14, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.L.; Elias, I.; Wright, R.; De Mello, A.; Cojocaru, D.; Dionne, J. Yield of diagnostic testing in evaluating etiology and end organ effects of pediatric hypertension. Pediatr. Nephrol. 2024, 39, 513–519. [Google Scholar] [CrossRef]
- Chrysaidou, K.; Chainoglou, A.; Karava, V.; Dotis, J.; Printza, N.; Stabouli, S. Secondary Hypertension in Children and Adolescents: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 37–44. [Google Scholar] [CrossRef]
- Roche, S.L.; Silversides, C.K. Hypertension, obesity, and coronary artery disease in the survivors of congenital heart disease. Can. J. Cardiol. 2013, 29, 841–848. [Google Scholar] [CrossRef]
- Gillett, C.; Wong, A.; Wilson, D.G.; Wolf, A.R.; Martin, R.P.; Kenny, D. Underrecognition of elevated blood pressure readings in children after early repair of coarctation of the aorta. Pediatr. Cardiol. 2011, 32, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Castaldi, B.; Baldini, L.; Gala, S.; del Gaizo, F.; D’Andrea, A.; Limongelli, G.; D’Aiello, A.F.; Scognamiglio, G.; Sarubbi, B.; et al. Masked hypertension in young patients after successful aortic coarctation repair: Impact on left ventricular geometry and function. J. Hum. Hypertens. 2011, 25, 739–745. [Google Scholar] [CrossRef]
- Canniffe, C.; Ou, P.; Walsh, K.; Bonnet, D.; Celermajer, D. Hypertension after repair of aortic coarctation—A systematic review. Int. J. Cardiol. 2013, 167, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Gidding, S.S.; Rocchini, A.P.; Moorehead, C.; Schork, M.A.; Rosenthal, A. Increased forearm vascular reactivity in patients with hypertension after repair of coarctation. Circulation 1985, 71, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Brili, S.; Dernellis, J.; Aggeli, C.; Pitsavos, C.; Hatzos, C.; Stefanadis, C.; Toutouzas, P. Aortic elastic properties in patients with repaired coarctation of aorta. Am. J. Cardiol. 1998, 82, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Perloff, J.K.; Bhuta, S.M.; Laks, H.; Drinkwater, D.C.; Child, J.S.; Miner, P.D. Structural abnormalities of great arterial walls in congenital heart disease: Light and electron microscopic analyses. Circulation 2001, 103, 393–400. [Google Scholar] [CrossRef]
- Ladouceur, M.; Boutouyrie, P.; Boudjemline, Y.; Khettab, H.; Redheuil, A.; Legendre, A.; Cohen, S.; Iserin, L.; Bonnet, D.; Mousseaux, E. Unknown complication of arterial switch operation: Resistant hypertension induced by a strong aortic arch angulation. Circulation 2013, 128, e466–e468. [Google Scholar] [CrossRef]
- Di Salvo, G.; Bulbul, Z.; Pergola, V.; Issa, Z.; Siblini, G.; Muhanna, N.; Galzerano, D.; Fadel, B.; Al Joufan, M.; Al Fayyadh, M.; et al. Gothic aortic arch and cardiac mechanics in young patients after arterial switch operation for d-transposition of the great arteries. Int. J. Cardiol. 2017, 241, 163–167. [Google Scholar] [CrossRef]
- Senzaki, H.; Iwamoto, Y.; Ishido, H.; Matsunaga, T.; Taketazu, M.; Kobayashi, T.; Asano, H.; Katogi, T.; Kyo, S. Arterial haemodynamics in patients after repair of tetralogy of Fallot: Influence on left ventricular after load and aortic dilatation. Heart 2008, 94, 70–74. [Google Scholar] [CrossRef]
- Daniels, S.R.; Loggie, J.M.; Schwartz, D.C.; Strife, J.L.; Kaplan, S. Systemic hypertension secondary to peripheral vascular anomalies in patients with Williams syndrome. J. Pediatr. 1985, 106, 249–251. [Google Scholar] [CrossRef]
- Giordano, R.; Forno, D.; Lanfranco, F.; Manieri, C.; Ghizzoni, L.; Ghigo, E. Metabolic and cardiovascular outcomes in a group of adult patients with Turner’s syndrome under hormonal replacement therapy. Eur. J. Endocrinol. 2011, 164, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, N.C.; Unwin, R.; Brook, C.G.; Hindmarsh, P.C. Blood pressure and Turner syndrome. Clin. Endocrinol. 2000, 52, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Niu, Z.; Huang, Q.; Sheng, W.; Wang, T. A meta-analysis of the incidence rate of postoperative acute kidney injury in patients with congenital heart disease. BMC Nephrol. 2020, 21, 350. [Google Scholar] [CrossRef] [PubMed]
- Madsen, N.L.; Goldstein, S.L.; Frøslev, T.; Christiansen, C.F.; Olsen, M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017, 92, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Irshad, M.; Parry, N.A. Pediatric hypertension: An updated review. Clin. Hypertens. 2020, 26, 22. [Google Scholar] [CrossRef] [PubMed]
- Wyszyńska, T.; Cichocka, E.; Wieteska-Klimczak, A.; Jobs, K.; Januszewicz, P. A single pediatric center experience with 1025 children with hypertension. Acta Paediatr. 1992, 81, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, Y.S.; Lee, S.T.; Cho, H. Pediatric renovascular hypertension: Treatment outcome according to underlying disease. Pediatr. Int. 2018, 60, 264–269. [Google Scholar] [CrossRef]
- Tullus, K.; Brennan, E.; Hamilton, G.; Lord, R.; McLaren, C.A.; Marks, S.D.; Roebuck, D.J. Renovascular hypertension in children. Lancet 2008, 371, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Stonebrook, E.; Kallash, M. Pediatric hypertension: Review of the definition, diagnosis, and initial management. Int. J. Pediatr. Adolesc. Med. 2022, 9, 1–6. [Google Scholar] [CrossRef]
- Bhavani, N. Pediatric endocrine hypertension. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. S4), S361–S366. [Google Scholar] [CrossRef]
- Gambelunghe, A.; Sallsten, G.; Borné, Y.; Forsgard, N.; Hedblad, B.; Nilsson, P.; Fagerberg, B.; Engström, G.; Barregard, L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016, 149, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.; Zhang, Y.; Solar-Yohay, S.; Shi, V. Clinical and demographic characteristics of children with hypertension. Hypertension 2012, 60, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- de Simone, G.; Mancusi, C.; Hanssen, H.; Genovesi, S.; Lurbe, E.; Parati, G.; Sendzikaite, S.; Valerio, G.; Di Bonito, P.; Di Salvo, G.; et al. Hypertension in children and adolescents. Eur. Heart J. 2022, 43, 3290–3301. [Google Scholar] [CrossRef] [PubMed]
- Dionne, J.M. Determinants of Blood Pressure in Neonates and Infants: Predictable Variability. Hypertension 2021, 77, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Dionne, J.M.; Abitbol, C.L.; Flynn, J.T. Hypertension in infancy: Diagnosis, management and outcome. Pediatr. Nephrol. 2012, 27, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Pejovic, B.; Peco-Antic, A.; Marinkovic-Eric, J. Blood pressure in non-critically ill preterm and full-term neonates. Pediatr. Nephrol. 2007, 22, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Altemose, K.; Dionne, J.M. Neonatal hypertension: Concerns within and beyond the neonatal intensive care unit. Clin. Exp. Pediatr. 2022, 65, 367–376. [Google Scholar] [CrossRef]
- Dionne, J.M.; Bremner, S.A.; Baygani, S.K.; Batton, B.; Ergenekon, E.; Bhatt-Mehta, V.; Dempsey, E.; Kluckow, M.; Pesco Koplowitz, L.; Apele-Freimane, D.; et al. Method of Blood Pressure Measurement in Neonates and Infants: A Systematic Review and Analysis. J. Pediatr. 2020, 221, 23–31.e5. [Google Scholar] [CrossRef]
- Harer, M.W.; Kent, A.L. Neonatal hypertension: An educational review. Pediatr. Nephrol. 2019, 34, 1009–1018. [Google Scholar] [CrossRef]
- Jetton, J.G.; Guillet, R.; Askenazi, D.J.; Dill, L.; Jacobs, J.; Kent, A.L.; Selewski, D.T.; Abitbol, C.L.; Kaskel, F.J.; Mhanna, M.J.; et al. Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a Retrospective Cohort Study. Front. Pediatr. 2016, 4, 68. [Google Scholar] [CrossRef]
- Starr, M.C.; Flynn, J.T. Neonatal hypertension: Cases, causes, and clinical approach. Pediatr. Nephrol. 2019, 34, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Verma, R.P. Neonatal Hypertension; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kamath, N.; Goud, B.R.; Phadke, K.D.; Iyengar, A. Use of oscillometric devices for the measurement of blood pressure-comparison with the gold standard. Indian J. Pediatr. 2012, 79, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Donahue, K.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; et al. Screening for high blood pressure in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. JAMA 2020, 324, 1878–1883. [Google Scholar] [PubMed]
- Edvardsson, V.O.; Steinthorsdottir, S.D.; Eliasdottir, S.B.; Indridason, O.S.; Palsson, R. Birth weight and childhood blood pressure. Curr. Hypertens. Rep. 2012, 14, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.D.; Meyer, R.A.; Loggie, J.M. Determinants of cardiac involvement in children and adolescents with essential hypertension. Circulation 1990, 82, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Gu, D.; Chen, J.; Huang, J.F.; Cao, J.; Chen, J.C.; Li, J.; Lu, F.; Mu, J.; Ma, J.; et al. Physical activity reduces salt sensitivity of blood pressure: The Genetic Epidemiology Network of Salt Sensitivity study. Am. J. Epidemiol. 2012, 176 (Suppl. S7), S106–S113. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.E.; van Eijsden, M.; Stronks, K.; Gemke, R.J.; Vrijkotte, T.G. The association between prenatal psychosocial stress and blood pressure in the child at age 5–7 years. PLoS ONE 2012, 7, e43548. [Google Scholar] [CrossRef] [PubMed]
- Wiesen, J.; Adkins, M.; Fortune, S.; Horowitz, J.; Pincus, N.; Frank, R.; Vento, S.; Hoffman, C.; Goilav, B.; Trachtman, H. Evaluation of pediatric patients with mild-to-moderate hypertension: Yield of diagnostic testing. Pediatrics 2008, 122, e988–e993. [Google Scholar] [CrossRef] [PubMed]
- Killian, L.; Simpson, J.M.; Savis, A.; Rawlins, D.; Sinha, M.D. Electrocardiography is a poor screening test to detect left ventricular hypertrophy in children. Arch. Dis. Child. 2010, 95, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.; Prokupetz, A.; KorenMorag, N.; Grossman, E.; Shamiss, A. Comparison of usefulness of Sokolow and Cornell criteria for left ventricular hypertrophy in subjects aged <20 years versus >30 years. Am. J. Cardiol. 2012, 110, 440–444. [Google Scholar]
- Armstrong, A.C.; Gidding, S.; Gjesdal, O.; Wu, C.; Bluemke, D.A.; Lima, J.A. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc. Imaging 2012, 5, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Castelli, P.K.; Dillman, J.R.; Kershaw, D.B.; Khalatbari, S.; Stanley, J.C.; Smith, E.A. Renal sonography with Doppler for detecting suspected pediatric renin-mediated hypertension—Is it adequate? Pediatr. Radiol. 2014, 44, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Cifkova, R.; Cruickshank, J.K.; Dillon, M.J.; Ferreira, I.; Invitti, C.; Kuznetsova, T.; Laurent, S.; Mancia, G.; Morales-Olivas, F.; et al. Manejo de la hipertensión arterial en niños y adolescentes: Recomendaciones de la Sociedad Europea de Hipertensión [Management of high blood pressure in children and adolescents: Recommendations of the European Society of hypertension]. An. Pediatr. 2010, 73, 51.e1–51.e28. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Damasceno, M.M.; de Araújo, M.F.; de Freitas, R.W.; de Almeida, P.C.; Zanetti, M.L. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice—An exploratory study. J. Clin. Nurs. 2011, 20, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Torrance, B.; McGuire, K.A.; Lewanczuk, R.; McGavock, J. Overweight, physical activity and high blood pressure in children: A review of the literature. Vasc. Health Risk Manag. 2007, 3, 139–149. [Google Scholar] [PubMed]
- Gregoski, M.J.; Barnes, V.A.; Tingen, M.S.; Harshfield, G.A.; Treiber, F.A. Breathing awareness meditation and LifeSkills Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J. Adolesc. Health 2011, 48, 59–64. [Google Scholar] [CrossRef]
- Sorof, J.M.; Cargo, P.; Graepel, J.; Humphrey, D.; King, E.; Rolf, C.; Cunningham, R.J. Beta-blocker/thiazide combination for treatment of hypertensive children: A randomized double-blind, placebo-controlled trial. Pediatr. Nephrol. 2002, 17, 345–350. [Google Scholar] [CrossRef]
- Burrello, J.; Erhardt, E.M.; Saint-Hilary, G.; Veglio, F.; Rabbia, F.; Mulatero, P.; Monticone, S.; D’Ascenzo, F. Pharmacological Treatment of Arterial Hypertension in Children and Adolescents: A Network Meta-Analysis. Hypertension 2018, 72, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Berezny, K.Y.; Kilaru, R.; Benjamin, D.K.J.R.; Kay, J.D.; Hazan, L.; Portman, R.; Hogg, R.; Deitchman, D.; Califf, R.M.; et al. Racial differences are seen in blood pressure response to fosinopril in hypertensive children. Am. Heart J. 2006, 152, 394–399. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Karpettas, N.; Kapoyiannis, A.; Stefanidis, C.J.; Vazeou, A. Home blood pressure monitoring in children and adolescents: A systematic review. J. Hypertens. 2009, 27, 1941–1947. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avesani, M.; Calcaterra, G.; Sabatino, J.; Pelaia, G.; Cattapan, I.; Barillà, F.; Martino, F.; Pedrinelli, R.; Bassareo, P.P.; Di Salvo, G., on behalf of the Working Group on Congenital Heart Disease, Cardiovascular Prevention in Paediatric Age of the Italian Society of Cardiology (SIC). Pediatric Hypertension: A Condition That Matters. Children 2024, 11, 518. https://doi.org/10.3390/children11050518
Avesani M, Calcaterra G, Sabatino J, Pelaia G, Cattapan I, Barillà F, Martino F, Pedrinelli R, Bassareo PP, Di Salvo G on behalf of the Working Group on Congenital Heart Disease, Cardiovascular Prevention in Paediatric Age of the Italian Society of Cardiology (SIC). Pediatric Hypertension: A Condition That Matters. Children. 2024; 11(5):518. https://doi.org/10.3390/children11050518
Chicago/Turabian StyleAvesani, Martina, Giuseppe Calcaterra, Jolanda Sabatino, Giulia Pelaia, Irene Cattapan, Francesco Barillà, Francesco Martino, Roberto Pedrinelli, Pier Paolo Bassareo, and Giovanni Di Salvo on behalf of the Working Group on Congenital Heart Disease, Cardiovascular Prevention in Paediatric Age of the Italian Society of Cardiology (SIC). 2024. "Pediatric Hypertension: A Condition That Matters" Children 11, no. 5: 518. https://doi.org/10.3390/children11050518