Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities
Abstract
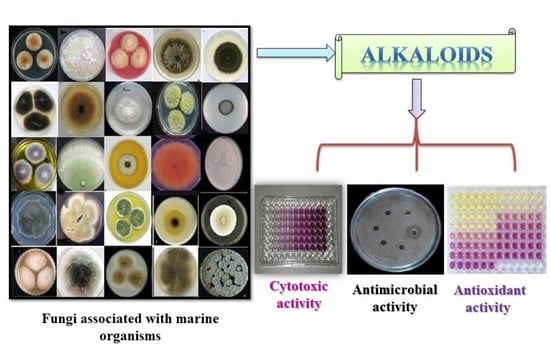
:1. Introduction
2. Classification of Different Classes of Alkaloids According to the Diverse Genera of the Marine-Associated Fungi in an Alphabetical Order
2.1. Acrostalagmus
2.2. Arthrinium
2.3. Auxarthron
2.4. Chaetomium
2.5. Cladosporium
2.6. Coniothyrium
2.7. Curvularia
2.8. Dichotomomyces
2.9. Eurotium
2.10. Eutypella
2.11. Exophiala
2.12. Fusarium
2.13. Hypocrea
2.14. Microsphaeropsis
2.15. Microsporum
2.16. Neosartorya
2.17. Nigrospora
2.18. Paecilomyces
2.19. Penicillium
2.20. Pleosporales
2.21. Pseudallescheria
2.22. Scedosporium
2.23. Scopulariopsis
2.24. Stagonosporopsis
2.25. Thielavia
2.26. Westerdykella
2.27. Xylariaceae
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Kashef, D.H.; Youssef, F.S.; Hartmann, R.; Knedel, T.-O.; Janiak, C.; Lin, W.; Reimche, I.; Teusch, N.; Liu, Z.; Proksch, P. Azaphilones from the Red Sea fungus Aspergillus falconensis. Mar. Drugs 2020, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- El-Kashef, D.H.; Youssef, F.S.; Reimche, I.; Teusch, N.; Müller, W.E.; Lin, W.; Frank, M.; Liu, Z.; Proksch, P. Polyketides from the marine-derived fungus Aspergillus falconensis: In silico and in vitro cytotoxicity studies. Bioorg. Med. Chem. 2020, 115883. [Google Scholar] [CrossRef]
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive alkaloids from Genus Aspergillus: Mechanistic interpretation of their antimicrobial and potential SARS-CoV-2 inhibitory activity using molecular modelling. Int. J. Mol. Sci. 2021, 22, 1866. [Google Scholar] [CrossRef]
- Youssef, F.S.; Singab, A.N.B. An updated review on the secondary metabolites and biological activities of Aspergillus ruber and Aspergillus flavus and exploring the cytotoxic potential of their isolated compounds using virtual screening. Evid.-Based Complementary Altern. Med. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, T.; De Mol, M.L.; De Bruycker, A.; De Maeseneire, S.L.; Soetaert, W.K. Alkaloids from marine fungi: Promising antimicrobials. Antibiotics 2020, 9, 340. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs. 2016, 14, 76. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Deng, M.; Zhou, Q.; Wang, J.; Liu, J.; Chen, C.; Qi, C.; Luo, Z.; Xue, Y. Asperversiamides, Linearly fused prenylated indole alkaloids from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Han, Z.; Peng, J.; Qian, P.-Y.; Qi, S.-H. Antifouling indole alkaloids from two marine derived fungi. Nat. Prod. Com. 2013, 8, 1934578X1300800313. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Chen, C.; Tao, H.; Lin, X.; Yang, B.; Zhou, X.; Liu, Y. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor SCSIO 41016. Org. Chem. Front. 2019, 6, 736–740. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Huang, Z.; Shi, X.-F.; Chen, Y.-C.; Zhang, W.-M.; Tian, X.-P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhai, H.; Zhu, K.; Yu, J.-H.; Zhang, Y.; Wang, Y.; Jiang, C.-S.; Zhang, X.; Zhang, Y.; Zhang, H. Bioactive pyridone alkaloids from a deep-sea-derived fungus Arthrinium sp. UJNMF0008. Mar. Drugs 2018, 16, 174. [Google Scholar] [CrossRef] [Green Version]
- Elsebai, M.F.; Rempel, V.; Schnakenburg, G.; Kehraus, S.; Müller, C.E.; König, G.M. Identification of a potent and selective cannabinoid CB1 receptor antagonist from Auxarthron reticulatum. ACS Med. Chem. Lett. 2011, 2, 866–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Min, X.; Huang, J.; Zhong, Y.; Wu, Y.; Li, X.; Deng, Y.; Jiang, Z.; Shao, Z.; Zhang, L. Cytoglobosins H and I, new antiproliferative cytochalasans from deep-sea-derived fungus Chaetomium globosum. Mar. Drugs 2016, 14, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Hua, C.; Yang, Y.; Dou, H.; Li, E.; Tan, R.; Hou, Y. Chaeoglobosin Fex inhibits poly (I: C)-induced activation of bone marrow-derived dendritic cells. Mol. Immunol. 2012, 51, 150–158. [Google Scholar] [CrossRef]
- Dou, H.; Song, Y.; Liu, X.; Gong, W.; Li, E.; Tan, R.; Hou, Y. Chaetoglobosin Fex from the marine-derived endophytic fungus inhibits induction of inflammatory mediators via Toll-like receptor 4 signaling in macrophages. Biol. Pharm. Bull. 2011, 34, 1864–1873. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Ge, X.; Mudassir, S.; Zhou, L.; Yu, G.; Che, Q.; Zhang, G.; Peng, J.; Gu, Q.; Zhu, T. New glutamine-containing azaphilone alkaloids from deep-sea-derived fungus Chaetomium globosum HDN151398. Mar. Drugs 2019, 17, 253. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.H.; Nenkep, V.N.; Siwe, X.N.; Leutou, A.S.; Feng, Z.; Zhang, D.; Choi, H.D.; Kang, J.S.; Son, B.W. An acetophenone derivative, clavatol, and a benzodiazepine alkaloid, circumdatin A, from the marine-derived fungus Cladosporium. Nat. Prod. Sci. 2009, 15, 130–133. [Google Scholar]
- Edrada-Ebel, R.; Jaspars, M. The 9th European conference on marine natural products. Mar. Drugs 2015, 13, 7150–7249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Jiao, R.-H.; Yao, L.-Y.; Han, W.-B.; Lu, Y.-H.; Tan, R.-X. Control of fungal morphology for improved production of a novel antimicrobial alkaloid by marine-derived fungus Curvularia sp. IFB-Z10 under submerged fermentation. Process. Biochem. 2016, 51, 185–194. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Xu, M.-Y.; Li, H.-J.; Zeng, K.-J.; Ma, W.-Z.; Tian, G.-B.; Xu, J.; Yang, D.-P.; Lan, W.-J. Diverse secondary metabolites from the marine-derived fungus Dichotomomyces cejpii F31-1. Mar. Drugs 2017, 15, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.-L.; Li, H.-J.; Smith, D.; Jaratsittisin, J.; Xia-Ke-Er, X.-F.-K.; Ma, W.-Z.; Guo, Y.-W.; Dong, J.; Shen, J.; Yang, D.-P. Polyketides and alkaloids from the marine-derived fungus Dichotomomyces cejpii F31-1 and the antiviral activity of scequinadoline A against dengue virus. Mar. Drugs 2018, 16, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming-he, L.; Hong-bo, H.; Lai-chun, L.; Jian-hua, J. Diketopiperazine Indole Alkaloids from a marine-derived fungus Eurotium amstelodami SCSIO 151. Nat. Prod. Res. Develop. 2015, 1, 50–54. [Google Scholar]
- Du, F.-Y.; Li, X.-M.; Li, C.-S.; Shang, Z.; Wang, B.-G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Du, F.-Y.; Li, X.-M.; Pedpradab, P.; Xu, G.-M.; Wang, B.-G. Rubrumazines A–C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef]
- Yan, H.J.; Li, X.M.; Li, C.S.; Wang, B.G. Alkaloid and anthraquinone derivatives produced by the marine-derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2012, 95, 163–168. [Google Scholar] [CrossRef]
- Li, D.-L.; Li, X.-M.; Proksch, P.; Wang, B.-G. 7-O-Methylvariecolortide A, a new spirocyclic diketopiperazine alkaloid from a marine mangrove derived endophytic fungus, Eurotium rubrum. Nat. Prod. Com. 2010, 5, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Wang, B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta 2008, 91, 1888–1893. [Google Scholar] [CrossRef]
- Staat, K.; Segatore, M. The phenomenon of chemo brain. Clin. J. Oncol. Nurs. 2005, 9, 713–721. [Google Scholar] [CrossRef]
- Youssef, F.S.; Menze, E.T.; Ashour, M.L. A potent lignan from Prunes alleviates inflammation and oxidative stress in lithium/pilocarpine-induced epileptic seizures in rats. Antioxidants 2020, 9, 575. [Google Scholar] [CrossRef]
- Zhong, W.-M.; Wang, J.-F.; Shi, X.-F.; Wei, X.-Y.; Chen, Y.-C.; Zeng, Q.; Xiang, Y.; Chen, X.-Y.; Tian, X.-P.; Xiao, Z.-H. Eurotiumins A–E, five new alkaloids from the marine-derived fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16, 136. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Wang, J.; Wei, X.; Fu, T.; Chen, Y.; Zeng, Q.; Huang, Z.; Huang, X.; Zhang, W.; Zhang, S. Three pairs of new spirocyclic alkaloid enantiomers from the marine-derived fungus Eurotium sp. SCSIO F452. Front. Chem. 2019, 7, 350. [Google Scholar] [CrossRef]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines A–M, thiodiketopiperazine-type alkaloids from deep sea derived fungus Eutypella sp. MCCC 3A00281. RSC Adv. 2017, 7, 33580–33590. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Satake, M.; Fukuzawa, S.; Sugahara, K.; Niitsu, A.; Shirai, T.; Tachibana, K. Two new indole alkaloids, 2-(3, 3-dimethylprop-1-ene)-costaclavine and 2-(3,3-dimethylprop-1-ene)-epicostaclavine, from the marine-derived fungus Aspergillus fumigatus. J. Nat. Med. 2012, 66, 222–226. [Google Scholar] [CrossRef]
- Nenkep, V.; Yun, K.; Son, B.W. Oxysporizoline, an antibacterial polycyclic quinazoline alkaloid from the marine-mudflat-derived fungus Fusarium oxysporum. J. Antibiot. 2016, 69, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Z.-L.; Wang, Y.; Tian, L.; Pei, Y.-H.; Hua, H.-M. A new alkaloid from the marine-derived fungus Hypocrea virens. Nat. Prod. Res. 2011, 25, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Cai, S.-Y.; Hao, X.-M.; Cao, F.; Zhu, H.-J. Alkaloids and butyrolactones from a marine-derived Microsphaeropsis sp. fungus. Chem. Nat. Comp. 2018, 54, 402–404. [Google Scholar] [CrossRef]
- Wijesekara, I.; Li, Y.-X.; Vo, T.-S.; Van Ta, Q.; Ngo, D.-H.; Kim, S.-K. Induction of apoptosis in human cervical carcinoma HeLa cells by neoechinulin A from marine-derived fungus Microsporum sp. Process. Biochem. 2013, 48, 68–72. [Google Scholar] [CrossRef]
- Wu, B.; Chen, G.; Liu, Z.-g.; Pei, Y. Two new alkaloids from a marine-derived fungus Neosartorya fischeri. Rec. Nat. Prod. 2015, 9, 271–275. [Google Scholar]
- Kumla, D.; Shine Aung, T.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Inácio, Â.; Costa, P.M.; Lee, M.; Sekeroglu, N. A new dihydrochromone dimer and other secondary metabolites from cultures of the marine sponge-associated fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213. Mar. Drugs 2017, 15, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Cao, Y.; Wang, L.; Chen, G.; Pei, Y.-H. New alkaloids from a marine-derived fungus Neosartorya sp. HN-M-3. J. Asian Nat. Prod. Res. 2013, 15, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-J.; Bao, J.; Zhang, X.-Y.; Xu, X.-Y.; Nong, X.-H.; Qi, S.-H. Alkaloids and citrinins from marine-derived fungus Nigrospora oryzae SCSGAF 0111. Tetrahedron Lett. 2014, 55, 2749–2753. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.-M.; Wang, J.-N.; Li, X.; Wang, B.-G. Prenylated indole alkaloids from the marine-derived fungus Paecilomyces variotii. Chin. Chem. Lett. 2015, 26, 313–316. [Google Scholar] [CrossRef]
- Zhang, P.; Mandi, A.; Li, X.-M.; Du, F.-Y.; Wang, J.-N.; Li, X.; Kurtan, T.; Wang, B.-G. Varioxepine A, a 3 H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef]
- Pang, X.; Cai, G.; Lin, X.; Salendra, L.; Zhou, X.; Yang, B.; Wang, J.; Wang, J.; Xu, S.; Liu, Y. New alkaloids and polyketides from the marine sponge-derived fungus Penicillium sp. SCSIO41015. Mar. Drugs 2019, 17, 398. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Li, D.; Zhu, T.; Cai, S.; Wang, F.; Xiao, X.; Gu, Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Du, L.; Yang, X.; Zhu, T.; Wang, F.; Xiao, X.; Park, H.; Gu, Q. Diketopiperazine alkaloids from a deep ocean sediment derived fungus Penicillium sp. Chem. Pharm. Bull. 2009, 57, 873–876. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Zhang, L.; Zhu, T.; Gu, Q.; Li, D. Unusual pyrrolyl 4-quinolinone alkaloids from the marine-derived fungus Penicillium sp. ghq208. Chem. Pharm. Bull. 2012, 60, 1458–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; El Sayed, K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. MedChemComm 2013, 4, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ko, S.-K.; Son, S.; Shin, K.-S.; Ryoo, I.-J.; Hong, Y.-S.; Oh, H.; Hwang, B.Y.; Hirota, H.; Takahashi, S. Haenamindole, an unusual diketopiperazine derivative from a marine-derived Penicillium sp. KCB12F005. Bioorg. Med. Chem. Lett. 2015, 25, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.N.; Berdyshev, D.V.; Smetanina, O.F.; Ivanets, E.V.; Zhuravleva, O.I.; Rasin, A.B.; Khudyakova, Y.V.; Popov, R.S.; Dyshlovoy, S.A.; von Amsberg, G. Citriperazines AD produced by a marine algae-derived fungus Penicillium sp. KMM 4672. Nat. Prod. Res. 2020, 34, 1118–1123. [Google Scholar] [CrossRef]
- Yang, B.; Sun, W.; Wang, J.; Lin, S.; Li, X.-N.; Zhu, H.; Luo, Z.; Xue, Y.; Hu, Z.; Zhang, Y. A new breviane spiroditerpenoid from the marine-derived fungus Penicillium sp. TJ403-1. Mar. Drugs 2018, 16, 110. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Ding, W.; Ma, Z. Mass spectrometric characteristics of prenylated indole derivatives from marine-derived Penicillium sp. NH-SL. Mar. Drugs 2017, 15, 86. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Yu, K.; Chen, C.; Guo, H.; Yang, N.; Gao, H.; Liu, X.; Liu, M.; Tong, Y. Quinazolin-4-one coupled with pyrrolidin-2-iminium alkaloids from marine-derived fungus Penicillium aurantiogriseum. Mar. Drugs 2012, 10, 1297–1306. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, J.; Wang, Z.; Lin, X.; Zhao, B.; Kaliaperumal, K.; Liao, X.; Tu, Z.; Li, J.; Xu, S. Structurally diverse secondary metabolites from a deep-sea-derived fungus Penicillium chrysogenum SCSIO 41001 and their biological evaluation. Fitoterapia 2017, 117, 71–78. [Google Scholar] [CrossRef]
- Tsuda, M.; Kasai, Y.; Komatsu, K.; Sone, T.; Tanaka, M.; Mikami, Y.; Kobayashi, J. Citrinadin A, a novel pentacyclic alkaloid from marine-derived fungus Penicillium citrinum. Org. Lett. 2004, 6, 3087–3089. [Google Scholar] [CrossRef]
- Tsuda, M.; Sasaki, M.; Mugishima, T.; Komatsu, K.; Sone, T.; Tanaka, M.; Mikami, Y.; Kobayashi, J. Scalusamides A−C, new pyrrolidine alkaloids from the marine-derived fungus Penicillium citrinum. J. Nat. Prod. 2005, 68, 273–276. [Google Scholar] [CrossRef]
- Mugishima, T.; Tsuda, M.; Kasai, Y.; Ishiyama, H.; Fukushi, E.; Kawabata, J.; Watanabe, M.; Akao, K.; Kobayashi, J. Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J. Org. Chem. 2005, 70, 9430–9435. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Tsuda, M.; Sekiguchi, M.; Mikami, Y.; Kobayashi, J. Perinadine A, a novel tetracyclic alkaloid from marine-derived fungus Penicillium citrinum. Org. Lett. 2005, 7, 4261–4264. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Zhou, T.; Zhao, Y.-Y.; Chen, L.; Gong, M.-W.; Xia, Q.-W.; Ying, M.-G.; Zheng, Q.-H.; Zhang, Q.-Q. Antitumor effects and related mechanisms of penicitrinine A, a novel alkaloid with a unique spiro skeleton from the marine fungus Penicillium citrinum. Mar. Drugs 2015, 13, 4733–4753. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ding, P.; Liang, Z.; Song, Y.; Liu, Y.; Chen, G.; Li, J.L. Activated production of silent metabolites from marine-derived fungus Penicillium citrinum. Fitoterapia 2018, 127, 207–211. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Nong, X.; Wei, X.; Qi, S. Oxindole alkaloids from the fungus Penicillium commune DFFSCS026 isolated from deep-sea-derived sediments. Tetrahedron 2015, 71, 610–615. [Google Scholar] [CrossRef]
- Fan, Y.-Q.; Li, P.-H.; Chao, Y.-X.; Chen, H.; Du, N.; He, Q.-X.; Liu, K.-C. Alkaloids with cardiovascular effects from the marine-derived fungus Penicillium expansum Y32. Mar. Drugs 2015, 13, 6489–6504. [Google Scholar] [CrossRef]
- Niu, S.; Wang, N.; Xie, C.-L.; Fan, Z.; Luo, Z.; Chen, H.-F.; Yang, X.-W. Roquefortine J, a novel roquefortine alkaloid, from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Antibiot. 2018, 71, 658–661. [Google Scholar] [CrossRef]
- Zhou, L.N.; Zhu, T.J.; Cai, S.X.; Gu, Q.Q.; Li, D.H. Three new indole-containing diketopiperazine alkaloids from a deep-ocean sediment derived fungus Penicillium griseofulvum. Helv. Chim. Acta 2010, 93, 1758–1763. [Google Scholar] [CrossRef]
- He, J.; Lion, U.; Sattler, I.; Gollmick, F.A.; Grabley, S.; Cai, J.; Meiners, M.; Schünke, H.; Schaumann, K.; Dechert, U. Diastereomeric Quinolinone alkaloids from the marine-derived fungus Penicillium janczewskii. J. Nat. Prod. 2005, 68, 1397–1399. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Shen, N.-X.; Liang, Z.-Y.; Shen, L.; Chen, M.; Wang, C.-Y. Paraherquamide J, a new prenylated indole alkaloid from the marine-derived fungus Penicillium janthinellum HK1-6. Nat. Prod. Res. 2020, 34, 378–384. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Kalinovsky, A.I.; Khudyakova, Y.V.; Pivkin, M.V.; Dmitrenok, P.S.; Fedorov, S.N.; Ji, H.; Kwak, J.-Y.; Kuznetsova, T.A. Indole alkaloids produced by a marine fungus isolate of Penicillium janthinellum Biourge. J. Nat. Prod. 2007, 70, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-L.; Li, D.-Y.; Xie, L.-R.; Wu, X.; Hua, H.-M.; Li, Z.-L. Novel decaturin alkaloids from the marine-derived fungus Penicillium oxalicum. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, C.S.; Li, X.M.; An, C.Y.; Wang, B.G. Prenylated indole alkaloid derivatives from marine sediment-derived fungus Penicillium paneum SD-44. Helv. Chim. Acta 2014, 97, 1440–1444. [Google Scholar] [CrossRef]
- Li, C.-S.; An, C.-Y.; Li, X.-M.; Gao, S.-S.; Cui, C.-M.; Sun, H.-F.; Wang, B.-G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Li, C.-W.; Gao, H.; Huang, X.-J.; Cui, C.-B. Penicimutamides D–E: Two new prenylated indole alkaloids from a mutant of the marine-derived Penicillium purpurogenum G59. RSC Adv. 2017, 7, 24718–24722. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-W.; Wu, C.-J.; Cui, C.-B.; Xu, L.-L.; Cao, F.; Zhu, H.-J. Penicimutamides A–C: Rare carbamate-containing alkaloids from a mutant of the marine-derived Penicillium purpurogenum G59. RSC Adv. 2016, 6, 73383–73387. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Hao, X.; Tan, J.; Li, F.; Qiao, X.; Chen, S.; Xiao, C.; Chen, M.; Peng, Z. Raistrickindole A, an anti-HCV oxazinoindole alkaloid from Penicillium raistrickii IMB17-034. J. Nat. Prod. 2019, 82, 1391–1395. [Google Scholar] [CrossRef]
- Asiri, I.A.; Badr, J.M.; Youssef, D.T. Penicillivinacine, antimigratory diketopiperazine alkaloid from the marine-derived fungus Penicillium vinaceum. Phytochem. Lett. 2015, 13, 53–58. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, A.; Cao, F.; Liu, Y.-F. Diketopiperazine alkaloids and steroids from a marine-derived Pleosporales sp. fungus. Chem. Nat. Comp. 2018, 54, 818–820. [Google Scholar] [CrossRef]
- Zhang, D.; Son, B. 12, 13-Dihydroxyfumitremorgin C, fumitremorgin C, and brevianamide F, antibacterial diketopiperazine alkaloids from the marine-derived fungus Pseudallescheria sp. Nat. Prod. Sci. 2007, 13, 251–254. [Google Scholar]
- Lan, W.-J.; Wang, K.-T.; Xu, M.-Y.; Zhang, J.-J.; Lam, C.-K.; Zhong, G.-H.; Xu, J.; Yang, D.-P.; Li, H.-J.; Wang, L.-Y. Secondary metabolites with chemical diversity from the marine-derived fungus Pseudallescheria boydii F19-1 and their cytotoxic activity. RSC Adv. 2016, 6, 76206–76213. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.-J.; Xu, M.-Y.; Ju, Y.-C.; Wang, L.-Y.; Xu, J.; Yang, D.-P.; Lan, W.-J. Pseudellones A–C, three alkaloids from the marine-derived fungus Pseudallescheria ellipsoidea F42–3. Org. Lett. 2015, 17, 5156–5159. [Google Scholar] [CrossRef]
- Huang, L.-H.; Xu, M.-Y.; Li, H.-J.; Li, J.-Q.; Chen, Y.-X.; Ma, W.-Z.; Li, Y.-P.; Xu, J.; Yang, D.-P.; Lan, W.-J. Amino acid-directed strategy for inducing the marine-derived fungus Scedosporium apiospermum F41–1 to maximize alkaloid diversity. Org. Lett. 2017, 19, 4888–4891. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z. Xanthones and sesquiterpene derivatives from a marine-derived fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.-L.; Xu, R.-F.; Wang, C.-Y.; Qian, P.-Y.; Wang, K.-L.; Wei, M.-Y. Potent antifouling marine dihydroquinolin-2 (1H)-one-containing alkaloids from the gorgonian coral-derived fungus Scopulariopsis sp. Mar. Biotechnol. 2015, 17, 408–415. [Google Scholar] [CrossRef]
- Haga, A.; Tamoto, H.; Ishino, M.; Kimura, E.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Pyridone alkaloids from a marine-derived fungus, Stagonosporopsis cucurbitacearum, and their activities against azole-resistant Candida albicans. J. Nat. Prod. 2013, 76, 750–754. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. New production of antibacterial polycyclic quinazoline alkaloid, thielaviazoline, from anthranilic acid by the marine-mudflat-derived fungus Thielavia sp. Nat. Prod. Sci. 2016, 22, 216–219. [Google Scholar] [CrossRef]
- Xu, D.; Luo, M.; Liu, F.; Wang, D.; Pang, X.; Zhao, T.; Xu, L.; Wu, X.; Xia, M.; Yang, X. Cytochalasan and tyrosine-derived alkaloids from the marine sediment-derived fungus Westerdykella dispersa and their bioactivities. Sci. Rep. 2017, 7, 11956. [Google Scholar] [CrossRef] [Green Version]
- Nong, X.-H.; Zhang, X.-Y.; Xu, X.-Y.; Sun, Y.-L.; Qi, S.-H. Alkaloids from Xylariaceae sp., a marine-derived fungus. Nat. Prod. Commun. 2014, 9, 467–468. [Google Scholar] [CrossRef] [Green Version]


















| Compound | Genus | Biological Activity | References |
|---|---|---|---|
| Luteoalbusin A (1) and B (2) | Acrostalagmus |
| [14] |
| Compounds (3–5) | Acrostalagmus | ||
| Arthpyrones F–I (13–16) | Arthrinium |
| [15] |
| Apiosporamide (19) | Arthrinium |
| |
| Amauromine (21) | Auxarthron |
| [16] |
| Chaetoglobosin E (26) | Chaetomium |
| [17] |
| ChaetoglobosinFex (28) | Chaetomium |
| [19] |
| N-glutarylchaetoviridin C (33) | Chaetomium |
| [20] |
| Circumdatin A (34) | Cladosporium |
| [21] |
| (−)-Cereolactam (35) | Coniothyrium |
| [16] |
| (−)-Cereoaldomine (36) | Coniothyrium | ||
| Curvulamine (37) | Curvularia |
| [23] |
| Dichotomocej A (38) | Dichotomomyces |
| [24] |
| Scequinadoline A (57) | Dichotomomyces |
| [25] |
| Variecolorin G (59) | Eurotium |
| [29] [34] |
| Isoechinulin B (60) | Eurotium |
| [32] |
| Neoechinulin B (61) | Eurotium |
| [10] |
| Cristatumin A (62) | Eurotium |
| [27] |
| Cristatumin B (63) | Eurotium |
| [27] |
| Rubrumazines A–C (66–68) | Eurotium |
| [28] |
| Compounds (69–80) | Eurotium |
| [28] |
| 12-Demethyl-12 oxoeurotechinulin B (81) | Eurotium |
| [29] |
| Alkaloid E-7 (85) | Eurotium |
| [29] |
| Dehydroechinulin (93) | Eurotium |
| [31] |
| Dehydrovariecolorin L (94) | Eurotium |
| [31] |
| Echinuline (95) | Eurotium |
| [32] [33] [34] |
| Isoechinulin A (96) | Eurotium |
| [10] [34] |
| Dihydroxyisoechinulin A (98) | Eurotium |
| [33] |
| Neoechinulin E (100) | Eurotium |
| [31] |
| Cryptoechinuline D (101) | Eurotium |
| [31] |
| Rubrumline D (105) | Eurotium |
| [10] [32] |
| Variecolorine O (118) | Eurotium |
| [10] [34] |
| Neoechinulin C (122) | Eurotium |
| [10] |
| Isoechinulin D (123) and C (124) | Eurotium |
| [32] |
| Alkaloid E-7 (125) | Eurotium |
| [32] |
| Cryptoechinulin G (126) | Eurotium |
| [32] |
| Eurotiumins A–B (129–130) | Eurotium |
| [34] |
| Eurotiumins C (131) and E (133) | Eurotium |
| [34] |
| Eurotinoids A–C (134–136) | Eurotium |
| [34] |
| Dihydrocryptoechinulin D (137) | Eurotium |
| [34] |
| Eutypellazines A–M (138–150) | Eutypella |
| [36] |
| Circumdatin I (151) | Exophiala |
| [37] |
| Oxysporizoline (154) | Fusarium |
| [38] |
| Tryptoquivaline T (159) | Neosartorya |
| [44] |
| Tryptoquivaline U (160) | Neosartorya |
| [44] |
| Fiscalin B (161) | Neosartorya |
| [44] |
| Dihydrocarneamide A (169) | Paecilomyces |
| [46] |
| Iso-notoamide B (170) | Paecilomyces |
| [46] |
| Varioxepine A (171) | Paecilomyces |
| [47] |
| Meleagrin B (178) | Penicillium |
| [49] |
| Meleagrin (186) | Penicillium |
| [68] |
| Brevicompanine E (189) and H (192) | Penicillium |
| [49] |
| Methyl penicinoline (196) | Penicillium |
| [51] |
| Penicinoline (197) | Penicillium |
| [51] |
| Penitrems A, B, D, E and F (199–203)Paspaline (204) | Penicillium |
| [52] |
| 6-Bromopenitrem B (205) and E (206) | Penicillium |
| [52] |
| Auranomides A–C (216–218) | Penicillium |
| [57] |
| Scalusamides A–C (222–224) | Penicillium |
| [59,60,61,62] |
| Penicitrinine A (227) | Penicillium |
| [63] |
| Aluminiumneohydroxyaspergillin (233) | Penicillium |
| [65] |
| Communesin I (243) | Penicillium |
| [67] |
| Fumiquinazoline Q (244) | Penicillium | ||
| Communesin A–B (245–246) | Penicillium | ||
| Protuboxepin A–B (247–248) | Penicillium | ||
| Prelapatin B (249) | Penicillium | ||
| Glyantrypine (250) | Penicillium | ||
| Variecolorins M–O (255–257) | Penicillium |
| [69] |
| 3R*,4R*dihydroxy-4-(4′-methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone (260) | Penicillium |
| [70] |
| Shearinines D–E (268–269) and A (271) | Penicillium |
| [72] |
| Penipaline B and C (279–280) | Penicillium |
| [74] |
| Penipanoid A (283) and C (285) | Penicillium |
| [75] |
| Raistrickindole A (293) | Penicillium |
| [78] |
| Raistrickin (294) | Penicillium |
| [78] |
| Penicillivinacine (297) | Penicillium |
| [79] |
| Terretrione A (298) | Penicillium |
| [79] |
| Brevianamide F (300) | PenicilliumPseudallescheria |
| [79,81] |
| α-Cyclopiazonic acid (301) | Penicillium |
| [79] |
| Pseuboydone C (305) | Pseudallescheria |
| [82] |
| Speradine C (311) | Pseudallescheria |
| [82] |
| 24,25-Dehydro-10,11-dihydro-20-hydroxyaflavinin (318) | Pseudallescheria |
| [82] |
| Scedapin C (324) | Scedosporium |
| [84] |
| Scequinadoline D (327) | Scedosporium |
| [84] |
| Scopulamide (328) | Scopulariopsis |
| [85] |
| Lumichrome (329) | Scopulariopsis |
| [85] |
| Aflaquinolone A, D, F and G (331–334) | Scopulariopsis |
| [86] |
| 6-Deoxyaflaquinolone E (335) | Scopulariopsis |
| [86] |
| Didymellamide A (336) | Stagonosporopsis |
| [87] |
| Thielaviazoline (340) | Thielavia |
| [88] |
| Gymnastatin Z (341) | Westerdykella |
| [89] |
| Compound (343) | Xylariaceae |
| [90] |
| Quinolactacin A1, A2and C1 (346–348) | Xylariaceae |
| [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, F.S.; Simal-Gandara, J. Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities. Biomedicines 2021, 9, 485. https://doi.org/10.3390/biomedicines9050485
Youssef FS, Simal-Gandara J. Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities. Biomedicines. 2021; 9(5):485. https://doi.org/10.3390/biomedicines9050485
Chicago/Turabian StyleYoussef, Fadia S., and Jesus Simal-Gandara. 2021. "Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities" Biomedicines 9, no. 5: 485. https://doi.org/10.3390/biomedicines9050485