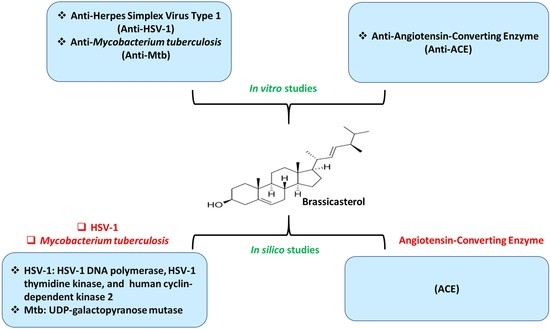
Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical In Vitro and In Silico Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antiherpetic Activity
2.1.1. Preparation of Vero Cells and the Virus
2.1.2. Cell Viability Assay for Determination of Cytotoxicity
2.1.3. Plaque Reduction Assay for Determination of Antiherpetic Activity
2.2. Antimycobacterial Assay
2.2.1. Bacterial Strains, Identification protocols, and Culturing Procedures
2.2.2. Drug Susceptibility Assay
2.3. Anti-Angiotensin-Converting Enzyme (ACE) Assay
2.4. In Silico Molecular Docking Analyses
3. Results and Discussion
3.1. Assessment of Cytotoxicity and Antiherpetic Properties
3.2. Assessment of Antituberculosis Activity
3.3. Evaluation of Anti-Angiotensin-Converting Enzyme Activity
3.4. Molecular Docking Evaluation
3.4.1. Brassicasterol with Enzymes Involved in Viral Replication
3.4.2. Brassicasterol with UDP-Galactopyranose Mutase
3.4.3. Brassicasterol with Angiotensin-Converting Enzyme
4. Conclusions
Funding
Conflicts of Interest
References
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M.; Masarčíková, R. Herpes simplex virus infection: An overview of the problem, pharmacologic therapy and dietary measures. Ceska Slov. Farm. 2017, 66, 95–102. [Google Scholar]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Widener, R.W.; Whitley, R.J. Herpes simplex virus. Handb. Clin. Neurol. 2014, 123, 251–263. [Google Scholar]
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. [Google Scholar] [CrossRef]
- Šudomová, M.; Shariati, M.A.; Echeverría, J.; Berindan-Neagoe, I.; Nabavi, S.M.; Hassan, S.T.S. A Microbiological, Toxicological, and Biochemical Study of the Effects of Fucoxanthin, a Marine Carotenoid, on Mycobacterium tuberculosis and the Enzymes Implicated in Its Cell Wall: A Link Between Mycobacterial Infection and Autoimmune Diseases. Mar. Drugs 2019, 17, 641. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Shingadia, D. The diagnosis of tuberculosis. Pediatr. Infect. Dis. J. 2012, 31, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Gowrishankar, S.; Rengasamy, K.R.R. Antimycobacterial, Enzyme Inhibition, and Molecular Interaction Studies of Psoromic Acid in Mycobacterium tuberculosis: Efficacy and Safety Investigations. J. Clin. Med. 2018, 7, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018, 73, 1138–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Fact Sheet on Tuberculosis (2020). Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 25 March 2020).
- Hassan, S.T.S.; Berchová-Bímová, K.; Šudomová, M.; Malaník, M.; Šmejkal, K.; Rengasamy, K.R.R. In Vitro Study of Multi-Therapeutic Properties of Thymus bovei Benth. Essential Oil and Its Main Component for Promoting Their Use in Clinical Practice. J. Clin. Med. 2018, 7, 283. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chang, P.; Wang, Q.; Hu, H.; Bai, F.; Li, N.; Yu, J. Effects of Angiotensin-Converting Enzyme Inhibitors on Arterial Stiffness: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cardiovasc. Ther. 2020, 2020, 7056184. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Carretero, O.A.; Vuljaj, N.; Liao, T.D.; Motivala, A.; Peterson, E.L.; Rhaleb, N.E. Angiotensin-converting enzyme inhibitors: A new mechanism of action. Circulation 2005, 112, 2436–2445. [Google Scholar] [CrossRef] [Green Version]
- Izzo, J.L., Jr.; Weir, M.R. Angiotensin-converting enzyme inhibitors. J. Clin. Hypertens. (Greenwich) 2011, 13, 667–675. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Popp, J.; Kölsch, H.; Friedrichs, S.; Jessen, F.; Stoffel-Wagner, B.; Bertsch, T.; Hartmann, T.; Maier, W.; von Bergmann, K.; et al. The plant sterol brassicasterol as additional CSF biomarker in Alzheimer’s disease. Acta Psychiatr. Scand. 2011, 124, 184–192. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Sahebkar, A.; Cosentino, T.; Pirro, M. Cholesterol-Lowering Nutraceuticals Affecting Vascular Function and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- CFR-Code Federal Regulations: Title 21 (2019). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.83 (accessed on 25 March 2020).
- Liland, N.S.; Pittman, K.; Whatmore, P.; Torstensen, B.E.; Sissener, N.H. Fucosterol Causes Small Changes in Lipid Storage and Brassicasterol Affects some Markers of Lipid Metabolism in Atlantic Salmon Hepatocytes. Lipids 2018, 53, 737–747. [Google Scholar] [CrossRef]
- Liland, N.S.; Espe, M.; Rosenlund, G.; Waagbø, R.; Hjelle, J.I.; Lie, Ø.; Fontanillas, R.; Torstensen, B.E. High levels of dietary phytosterols affect lipid metabolism and increase liver and plasma TAG in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2013, 110, 1958–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Laboratory Detection and Identification of Mycobacteria, 1st ed.; Approved Guideline; CLSI Document M48-A.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes, 2nd ed.; Approved Standard M24-A2; CLSI: Wayne, PA, USA, 2011. [Google Scholar]
- Hassan, S.T.S.; Švajdlenka, E. Biological Evaluation and Molecular Docking of Protocatechuic Acid from Hibiscus sabdariffa L. as a Potent Urease Inhibitor by an ESI-MS Based Method. Molecules 2017, 22, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, H.J.; Vere Hodge, R.A. Recent developments in anti-herpesvirus drugs. Br. Med. Bull. 2013, 106, 213–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.E.G.; Schluger, N.W. Advances in the diagnosis and treatment of latent tuberculosis infection. Curr. Opin. Infect. Dis. 2020, 33, 166–172. [Google Scholar] [CrossRef]
- Wächter, G.A.; Franzblau, S.G.; Montenegro, G.; Hoffmann, J.J.; Maiese, W.M.; Timmermann, B.N. Inhibition of Mycobacterium tuberculosis growth by saringosterol from Lessonia nigrescens. J. Nat. Prod. 2001, 64, 1463–1464. [Google Scholar] [CrossRef]
- Tan, M.A.; Takayama, H.; Aimi, N.; Kitajima, M.; Franzblau, S.G.; Nonato, M.G. Antitubercular triterpenes and phytosterols from Pandanus tectorius Soland. var. laevis. J. Nat. Med. 2008, 62, 232–235. [Google Scholar] [CrossRef]
- Olugbuyiro, J.A.; Moody, J.O.; Hamann, M.T. Phytosterols from Spondias mombin Linn with Antimycobacterial Activities. Afr. J. Biomed. Res. 2013, 16, 19–24. [Google Scholar]
- Chinsembu, K.C. Tuberculosis and nature’s pharmacy of putative anti-tuberculosis agents. Acta Trop. 2016, 153, 46–56. [Google Scholar] [CrossRef]
- Šudomová, M.; Hassan, S.T.S.; Khan, H.; Rasekhian, M.; Nabavi, S.M. A Multi-Biochemical and In Silico Study on Anti-Enzymatic Actions of Pyroglutamic Acid against PDE-5, ACE, and Urease Using Various Analytical Techniques: Unexplored Pharmacological Properties and Cytotoxicity Evaluation. Biomolecules 2019, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, H.; Wakita, K.; Inada, Y.; Hirose, S. Fucosterol decreases angiotensin converting enzyme levels with reduction of glucocorticoid receptors in endothelial cells. Biochem. Biophys. Res. Commun. 1986, 139, 348–352. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schang, L.M.; St Vincent, M.R.; Lacasse, J.J. Five years of progress on cyclin-dependent kinases and other cellular proteins as potential targets for antiviral drugs. Antivir. Chem. Chemother. 2006, 17, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Viegas, D.J.; Edwards, T.G.; Bloom, D.C.; Abreu, P.A. Virtual screening identified compounds that bind to cyclin dependent kinase 2 and prevent herpes simplex virus type 1 replication and reactivation in neurons. Antiviral. Res. 2019, 172, 104621. [Google Scholar] [CrossRef] [PubMed]
- Schang, L.M. The cell cycle, cyclin-dependent kinases, and viral infections: New horizons and unexpected connections. Prog. Cell. Cycle Res. 2003, 5, 103–124. [Google Scholar]
- Schang, L.M.; Phillips, J.; Schaffer, P.A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 1998, 72, 5626–5637. [Google Scholar] [CrossRef] [Green Version]
- Davido, D.J.; Leib, D.A.; Schaffer, P.A. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 2002, 76, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Schang, L.M.; Bantly, A.; Schaffer, P.A. Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdk2 and cdk4. J. Virol. 2002, 76, 7724–7735. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Kim, N.H. CDK2 Is Required for the DNA Damage Response During Porcine Early Embryonic Development. Biol. Reprod. 2016, 95, 31. [Google Scholar] [CrossRef]
- Tadesse, S.; Caldon, E.C.; Tilley, W.; Wang, S. Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J. Med. Chem. 2019, 62, 4233–4251. [Google Scholar] [CrossRef]
- Soltero-Higgin, M.; Carlson, E.E.; Gruber, T.D.; Kiessling, L.L. A unique catalytic mechanism for UDP-galactopyranose mutase. Nat. Struct. Mol. Biol. 2004, 11, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, K.A.; Besra, G.S. Mycobacterial cell wall biosynthesis: A multifaceted antibiotic target. Parasitology 2018, 145, 116–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natesh, R.; Schwager, S.L.; Evans, H.R.; Sturrock, E.D.; Acharya, K.R. Structural details on the binding of antihypertensive drugs captopril and enalaprilat to human testicular angiotensin I-converting enzyme. Biochemistry 2004, 43, 8718–8724. [Google Scholar] [CrossRef] [PubMed]




| Molecules | CC50 (μM) | IC50 (μM) | SI (CC50/IC50) |
|---|---|---|---|
| Brassicasterol | >50 | 1.2 ± 0.12 | >41.7 |
| Brassicasterol combined with ACV | >50 | 0.7 ± 0.24 | >71.4 |
| ACV (standard) | >50 | 2.1 ± 0.13 | >23.8 |
| Mycobacterial Strains | MIC (µM) | |
|---|---|---|
| Brassicasterol | Rifampicin | |
| Mtb a | 1.9 ± 0.12 | 0.1 ± 0.01 |
| Mtb-CI1 b | 2.0 ± 0.13 | 0.2 ± 0.02 |
| Mtb-CI2 b | 2.4 ± 0.15 | 0.2 ± 0.03 |
| Mtb-CI3 b | 2.2 ± 0.14 | 0.3 ± 0.02 |
| Mtb-CI4 b | 2.1 ± 0.14 | 0.3 ± 0.02 |
| Mtb-CI5 b | 1.9 ± 0.13 | 0.1 ± 0.02 |
| Mtb-CI6 b | 1.9 ± 0.13 | 0.3 ± 0.01 |
| Mtb-CI7 b | 2.1 ± 0.14 | 0.2 ± 0.01 |
| Mtb-CI8 b | 1.9 ± 0.11 | 0.4 ± 0.03 |
| Mtb-CI9 b | 2.1 ± 0.13 | 0.3 ± 0.02 |
| Mtb-CI10 b | 2.0 ± 0.15 | 0.1 ± 0.01 |
| Compounds | % Inhibition |
|---|---|
| Brassicasterol | 91.2 ± 0.43 |
| Captopril (standard) | 99.1 ± 0.12 |
| ACE-catalyzed reaction (no inhibition) | - |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.T.S. Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical In Vitro and In Silico Assessments. Biomedicines 2020, 8, 132. https://doi.org/10.3390/biomedicines8050132
Hassan STS. Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical In Vitro and In Silico Assessments. Biomedicines. 2020; 8(5):132. https://doi.org/10.3390/biomedicines8050132
Chicago/Turabian StyleHassan, Sherif T. S. 2020. "Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical In Vitro and In Silico Assessments" Biomedicines 8, no. 5: 132. https://doi.org/10.3390/biomedicines8050132