Anti-Inflammatory Effects of Diospyrin on Lipopolysaccharide-Induced Inflammation Using RAW 264.7 Mouse Macrophages
Abstract
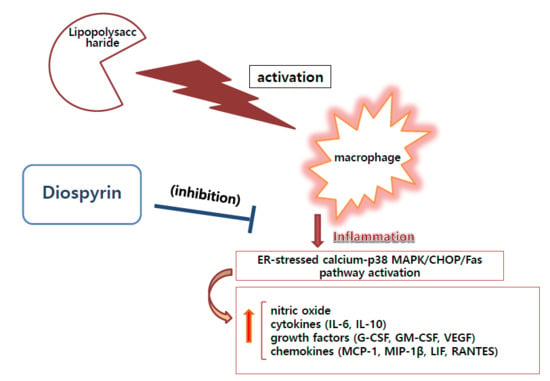
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Viability Assay
2.3. Quantification of NO Production
2.4. Intracellular Calcium Assay
2.5. Cytokines Production
2.6. RNA Isolation and Real Time RT-PCR Analysis
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. Effect of Diospyrin on Cell Viability
3.2. Effect of Diospyrin on NO Production
3.3. Effect of Diospyrin on Intracellular Calcium Release
3.4. Effect of Diospyrin on Cytokine Production
3.5. Effect of Diospyrin on mRNA Expression of CHOP and Fas
3.6. Effect of Diospyrin on Phosphorylation of p38 MAPK
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Capone, F.; Guerriero, E.; Colonna, G.; Maio, P.; Mangia, A.; Castello, G.; Costantini, S. Cytokinome Profile Evaluation in Patients with Hepatitis C Virus Infection. World J. Gastroenterol. 2014, 20, 9261–9269. [Google Scholar]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and Autoimmunity. Nat. Rev. Immunol. 2002, 2, 37–45. [Google Scholar] [CrossRef]
- Zhang, F.X.; Kirschning, C.J.; Mancinelli, R.; Xu, X.P.; Jin, Y.; Faure, E.; Mantovani, A.; Rothe, M.; Muzio, M.; Arditi, M. Bacterial Lipopolysaccharide Activates Nuclear Factor-kappaB through Interleukin-1 Signaling Mediators in Cultured Human Dermal Endothelial Cells and Mononuclear Phagocytes. J. Biol. Chem. 1999, 274, 7611–7614. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Hazra, B.; Mittra, B.; Das, A.; Majumder, H.K. Diospyrin, a Bisnaphthoquinone: A Novel Inhibitor of Type I DNA Topoisomerase of Leishmania Donovani. Mol. Pharmacol. 1998, 54, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-Inflammatory Effects of ScutellariaBaicalensis Water Extract on LPS-Activated RAW 264.7 Macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, W.; Yi, D.K. Immunostimulatory Effects of Gold Nanorod and Silica-Coated Gold Nanorod on RAW 264.7 Mouse Macrophages. Toxicol. Lett. 2012, 209, 51–57. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.J.; Lee, J.Y.; Kim, D.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effect of Baicalein on Polyinosinic-Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages. Viruses 2018, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effects of Angelica Sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide. Nutrients 2018, 10, 647. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef]
- Ferret, P.J.; Soum, E.; Negre, O.; Fradelizi, D. Auto-Protective Redox Buffering Systems in Stimulated Macrophages. BMC Immunol. 2002, 3, 3. [Google Scholar]
- Medina, E.A.; Morris, I.R.; Berton, M.T. Phosphatidylinositol 3-Kinase Activation Attenuates the TLR2-Mediated Macrophage Proinflammatory Cytokine Response to FrancisellaTularensis Live Vaccine Strain. J. Immunol. 2010, 185, 7562–7572. [Google Scholar] [CrossRef] [Green Version]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.S.; Zhan, D.; Chen, Y.L.; He, Y.; Liu, J.; Zhang, Z.J.; Sun, J.; et al. Resveratrol Inhibits LPS-Induced MAPKs Activation Via Activation of the Phosphatidylinositol 3-Kinase Pathway in Murine RAW 264.7 Macrophage Cells. PLoS ONE 2012, 7, e44107. [Google Scholar] [CrossRef]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible Nitric Oxide Synthase (iNOS) in Tumor Biology: The Two Sides of the Same Coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef]
- Thiemermann, C.; Vane, J. Inhibition of Nitric Oxide Synthesis Reduces the Hypotension Induced by Bacterial Lipopolysaccharides in the Rat in Vivo. Eur. J. Pharmacol. 1990, 182, 591–595. [Google Scholar] [CrossRef]
- Evans, T.; Carpenter, A.; Kinderman, H.; Cohen, J. Evidence of Increased Nitric Oxide Production in Patients with the Sepsis Syndrome. Circ. Shock 1993, 41, 77–81. [Google Scholar]
- Kankkunen, P.; Välimäki, E.; Rintahaka, J.; Palomäki, J.; Nyman, T.; Alenius, H.; Wolff, H.; Matikainen, S. TrichotheceneMycotoxins Activate NLRP3 Inflammasome through a P2X 7 Receptor and Src Tyrosine Kinase Dependent Pathway. Hum. Immunol. 2014, 75, 134–140. [Google Scholar] [CrossRef]
- Martin, B.N.; Wang, C.; Willette-Brown, J.; Herjan, T.; Gulen, M.F.; Zhou, H.; Bulek, K.; Franchi, L.; Sato, T.; Alnemri, E.S. IKKα Negatively Regulates ASC-Dependent Inflammasome Activation. Nat. Commun. 2014, 5, 4977. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Doi, S.; Nakashima, A.; Ueno, T.; Yokoyama, Y.; Kohno, N.; Masaki, T. Mizoribine Ameliorates Renal Injury and Hypertension Along with the Attenuation of Renal Caspase-1 Expression in Aldosterone-Salt-Treated Rats. PLoS ONE 2014, 9, e93513. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.A.; Lam, C.; Kashyap, P.; Salehi-Najafabadi, Z.; Singh, G.; Yu, Y. Analysis of the Cell Surface Expression of Cytokine Receptors using the Surface Protein Biotinylation Method. Methods Mol. Biol. 2014, 1172, 185–192. [Google Scholar]
- Gadient, R.A.; Patterson, P.H. Leukemia Inhibitory Factor, Interleukin 6, and Other Cytokines using the GP130 Transducing Receptor: Roles in Inflammation and Injury. Stem Cells 1999, 17, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, M.J.; Shen, F.; Smith, J.B.; Sharma, A.; Gaffen, S.L. Interleukin-17 Regulates Expression of the CXC Chemokine LIX/CXCL5 in Osteoblasts: Implications for Inflammation and Neutrophil Recruitment. J. Leukocyte Biol. 2004, 76, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Capelli, A.; Di Stefano, A.; Gnemmi, I.; Balbo, P.; Cerutti, C.; Balbi, B.; Lusuardi, M.; Donner, C. Increased MCP-1 and MIP-1β in Bronchoalveolar Lavage Fluid of Chronic Bronchitics. Eur. Respir. J. 1999, 14, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Qiu, Y.S.; Majumdar, S.; Gamble, E.; Matin, D.; Turato, G.; Fabbri, L.M.; Barnes, N.; Saetta, M.; Jeffery, P.K. Exacerbations of Bronchitis: Bronchial Eosinophilia and Gene Expression for Interleukin-4, Interleukin-5, and Eosinophil Chemoattractants. Am. J. Respir. Crit. Care Med. 2001, 164, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kyama, C.; Mihalyi, A.; Simsa, P.; Mwenda, J.; Tomassetti, C.; Meuleman, C.; D’Hooghe, T. Non-Steroidal Targets in the Diagnosis and Treatment of Endometriosis. Curr. Med. Chem. 2008, 15, 1006–1017. [Google Scholar] [CrossRef]
- Appelmann, I.; Liersch, R.; Kessler, T.; Mesters, R.M.; Berdel, W.E. Angiogenesis inhibition in cancer therapy: Platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) and their receptors: Biological functions and role in malignancy. Recent Results Cancer Res. 2010, 180, 51–81. [Google Scholar]
- Dace, D.S.; Khan, A.A.; Stark, J.L.; Kelly, J.; Cross, A.H.; Apte, R.S. Interleukin-10 Overexpression Promotes Fas-Ligand-Dependent Chronic Macrophage-Mediated Demyelinating Polyneuropathy. PLoS ONE 2009, 4, e7121. [Google Scholar] [CrossRef]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 Activation Causes Translocation of Bax to the Endoplasmic Reticulum during the Resolution of Airway Mucous Cell Hyperplasia by IFN-Gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N.Y. Acad. Sci. 2009, 1173 (Suppl. 1), E40. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Z.; Ron, D. Stress-Induced Phosphorylation and Activation of the Transcription Factor CHOP (GADD153) by p38 MAP Kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Name 1 | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| CHOP | CCACCACACCTGAAAGCAG | TCCTCATACCAGGCTTCCA |
| FAS | CGCTGTTTTCCCTTGCTG | CCTTGAGTATGAACTCTTAACTGTGAG |
| β-actin | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahidullah, A.; Lee, J.-Y.; Kim, Y.-J.; Halimi, S.M.A.; Rauf, A.; Kim, H.-J.; Kim, B.-Y.; Park, W. Anti-Inflammatory Effects of Diospyrin on Lipopolysaccharide-Induced Inflammation Using RAW 264.7 Mouse Macrophages. Biomedicines 2020, 8, 11. https://doi.org/10.3390/biomedicines8010011
Shahidullah A, Lee J-Y, Kim Y-J, Halimi SMA, Rauf A, Kim H-J, Kim B-Y, Park W. Anti-Inflammatory Effects of Diospyrin on Lipopolysaccharide-Induced Inflammation Using RAW 264.7 Mouse Macrophages. Biomedicines. 2020; 8(1):11. https://doi.org/10.3390/biomedicines8010011
Chicago/Turabian StyleShahidullah, Adnan, Ji-Young Lee, Young-Jin Kim, Syed Muhammad Ashhad Halimi, Abdur Rauf, Hyun-Ju Kim, Bong-Youn Kim, and Wansu Park. 2020. "Anti-Inflammatory Effects of Diospyrin on Lipopolysaccharide-Induced Inflammation Using RAW 264.7 Mouse Macrophages" Biomedicines 8, no. 1: 11. https://doi.org/10.3390/biomedicines8010011