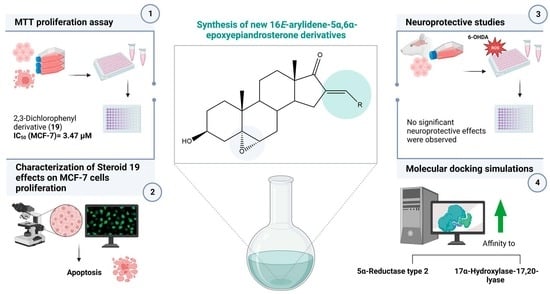
Synthesis, In Vitro Biological Evaluation of Antiproliferative and Neuroprotective Effects and In Silico Studies of Novel 16E-Arylidene-5α,6α-epoxyepiandrosterone Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. General Considerations
2.1.2. General Procedure to Prepare 16E-Arylidenedehydroepiandrosterone Derivatives (2–14)
2.1.3. General Procedure to Prepare 16E-Arylidene-5α,6α-epoxyepiandrosterone Derivatives (15–27)
2.2. Biological Evaluation
2.2.1. Cell Culture
2.2.2. Preparation of Compounds Solutions
2.2.3. MTT Cell Proliferation Assay
2.2.4. Immunocytochemistry Assay
2.2.5. PI Incorporation
2.2.6. Analysis of Cell Nuclear Morphology and Distribution with ImageJ
2.2.7. Neuroprotective Studies
2.2.8. Statistical Analysis
2.3. Molecular Docking Simulations
2.3.1. Preparation of Macromolecules and Ligands
2.3.2. Grid Map Parameters
2.3.3. Method Validation and Molecular Docking Simulations
3. Results and Discussion
3.1. Chemical Synthesis
3.2. Biological Evaluation
3.2.1. Antiproliferative Activity
3.2.2. Characterization of Steroid 19 Effects on MCF-7 Cells Proliferation
3.2.3. Study of Neuroprotective Effects on N27 Cells
3.3. Molecular Docking Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burger, A.; Abraham, D.J.; Rotella, D.P. Burger’s Medicinal Chemistry, Drug Discovery and Development; Wiley: Hoboken, NJ, USA, 2010; Volume 7, ISBN 9780470770085/0470770082/9780470278154/0470278153. [Google Scholar]
- Latham, K.A.; Zamora, A.; Drought, H.; Matejuk, A.; Offner, H.; Edward, F. Estradiol Treatment Redirects the Isotype of the Autoantibody Response and Prevents the Development of Autoimmune Arthritis. J. Immunol. 2003, 171, 5820–5827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, E.R. Minireview: Extranuclear Steroid Receptors: Roles in Modulation of Cell Functions. Mol. Endocrinol. 2011, 25, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.H.; Mir, B.P.; Lone, I.H.; Suri, K.A.; Kumar, H.M.S. Studies on Novel D-Ring Substituted Steroidal Pyrazolines as Potential Anticancer Agents. Steroids 2010, 75, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; Nafie, M.S.; Elmegeed, G.A.; Ali, I.A.I. Auspicious Role of the Steroidal Heterocyclic Derivatives as a Platform for Anti-Cancer Drugs. Bioorg. Chem. 2017, 73, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Terent, A.O.; Savidov, N.; Gloriozova, T.A. Hydroperoxy Steroids and Triterpenoids Derived from Plant and Fungi: Origin, Structures and Biological Activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Progress in Lipid Research Antitumor and Hepatoprotective Activity of Natural and Synthetic Neo Steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.; Fei, B.; Huang, G.; Diao, Q. Nature-Derived Anticancer Steroids Outside Cardica Glycosides. Fitoterapia 2020, 147, 104757. [Google Scholar] [CrossRef]
- Dubey, R.; Oparil, S.; Imthurn, B.; Jackson, E. Sex Hormones and Hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, P.J.; Blum, K.; Trachtenberg, M.C. Steroid Receptors and Disease: Cancer, Autoimmune, Bone, and Circulatory Disorders. Trends Pharmacol. Sci. 1988, 10, 122. [Google Scholar] [CrossRef]
- Holst, J.P.; Soldin, S.J.; Tractenberg, R.E.; Guo, T.; Kundra, P.; Verbalis, J.G.; Jonklaas, J.; Clinical, G. Use of Steroid Profiles in Determining the Cause of Adrenal Insufficiency. Steroids 2006, 72, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Jursic, B.S.; Kumar, S.; Creech, C.C.; Neumann, D.M. Novel and Efficient Synthesis and Antifungal Evaluation of 2,3-Functionalized Cholestane and Androstane Derivatives. Bioorg. Med. Chem. Lett. 2020, 20, 7372–7375. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Zargar, M.I.; Ganaie, B.A. Synthesis and Antimicrobial Studies of Chalconyl Pregnenolones. Steroids 2011, 76, 1358–1362. [Google Scholar] [CrossRef]
- Poirier, D.; Chang, H.; Azzi, A.; Boivin, R.P.; Lin, S. Estrone and Estradiol C-16 Derivatives as Inhibitors of Type 1 17b-Hydroxysteroid Dehydrogenase. Mol. Cell. Endocrinol. 2006, 248, 236–238. [Google Scholar] [CrossRef]
- Vosooghi, M.; Yahyavi, H.; Divsalar, K.; Shamsa, H.; Kheirollahi, A.; Safavi, M.; Ardestani, S.K.; Sadeghi-Neshat, S.; Mohammadhosseini, N.; Edraki, N.; et al. Synthesis and in Vitro Cytotoxic Activity Evaluation of (E)-16-(Substituted Benzylidene) Derivatives of Dehydroepiandrosterone. Daru 2013, 21, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, R.; Guleria, S.; Thota, S.; Hartmann, R.W.; Zimmer, C. Synthesis of Imidazole-Derived Steroidal Hybrids as Potent Aromatase Inhibitors. Med. Chem. Res. 2013, 22, 692–698. [Google Scholar] [CrossRef]
- Brito, V.; Alves, G.; Almeida, P.; Silvestre, S. Highlights on Steroidal Arylidene Derivatives as a Source of Pharmacologically Active Compounds: A Review. Molecules 2021, 26, 2032. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, Y.; Gao, P.; An, X.; Sun, Y.; Sun, X.; Hou, Y.; Shan, L. Synthesis and Anti-Inflammatory Activity Evaluation of 2-Dehydroepiandrosterone Benzene Methyl Derivatives. Chin. J. Org. Chem. 2019, 39, 2625–2631. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Fu, D.; Qin, T.; Ran, Y.; Xu, F.; Du, X. Discovery of Chalcone-Modified Estradiol Analogs as Antitumour Agents That Inhibit Tumour Angiogenesis and Epithelial to Mesenchymal Transition. Eur. J. Med. Chem. 2019, 176, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.S.; Silva, M.M.C.; Sa, M.L. Highly Efficient Epoxidation of Unsaturated Steroids Using Magnesium Bis(Monoperoxyphthalate) Hexahydrate. Tetrahedron 2009, 65, 2773–2781. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, X.; Gong, J.; Zhu, T.; Zhang, H.; Guo, Y. Synthesis and Biological Evaluation of 21-Arylidenepregnenolone Derivatives as Neuroprotective Agents. Bioorg. Med. Chem. Lett. 2012, 22, 2226–2229. [Google Scholar] [CrossRef]
- Chávez-Riveros, A.; Bratoeff, E.; Heuze, Y.; Soriano, J.; Moreno, I.; Sánchez-Márquez, A.; Cabeza, M. Synthesis and Identification of Pregnenolone Derivatives as Inhibitors of Isozymes of 5α-Reductase. Arch. Pharm. 2015, 348, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.; Ma, E. Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-Diene or 4,6-Diene 20-Ones and 4-Azasteroid 20-Oximes. Molecules 2012, 17, 355–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratoeff, E.; García, P.; Heuze, Y.; Soriano, J.; Mejía, A.; Labastida, A.M.; Valencia, N.; Cabeza, M. Molecular Interactions of Progesterone Derivatives with 5α-Reductase Types 1 and 2 and Androgen Receptors. Steroids 2010, 75, 499–505. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Li, C. Androsterone-Based Gels Enable Diastereospecific Reductions and Diastereoselective Epoxidations of Gelators. Org. Biomol. Chem. 2018, 16, 6791–6800. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective Assessment of Changes in Nuclear Morphology and Cell Distribution Following Induction of Apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristóvão, A.C.; Choi, D.-H.; Baltazar, G.; Beal, M.F.; Kim, Y.-S. The Role of NADPH Oxidase 1–Derived Reactive Oxygen Species in Paraquat-Mediated Dopaminergic Cell Death. Antioxid. Redox Signal. 2009, 11, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Brito, V.; Santos, A.O.; Almeida, P.; Silvestre, S. Novel 4-Azaandrostenes as Prostate Cancer Cell Growth Inhibitors: Synthesis, Antiproliferative Effects and Molecular Docking Studies. Comptes Rendus Chim. 2018, 22, 73–83. [Google Scholar] [CrossRef]
- Chattopadhaya, R.; Jindal, D.P.; Minu, M.; Gupta, R. Synthesis and Cytotoxic Studies of Hydroximino Derivatives of Some 16E-Arylidenosteroids. Arzneim. Forshung Drug Res. 2004, 556, 551–556. [Google Scholar] [CrossRef]
- Dubey, S.; Piplani, P.; Jindal, D.P. Synthesis and Evaluation of Some 16-Benzylidene Substituted 3,17-Dioximino Androstene Derivatives as Anticancer Agents. Lett. Drug Des. Discov. 2005, 2, 537–545. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Zhang, Z.; Yang, Z. Steroidal[17,16-d]Pyrimidines Derived from Dehydroepiandrosterone: A Convenient Synthesis, Antiproliferation Activity, Structure-Activity Relationships, and Role of Heterocyclic Moiety. Sci. Rep. 2017, 7, 44439. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Wu, H.; Yang, J.; Xiao, Y.; Altenbach, H.; Qiu, G. Synthesis, Characterization and Biological Evaluation of Some 16E-Arylidene Androstane Derivatives as Potential Anticancer Agents. Steroids 2011, 76, 709–723. [Google Scholar] [CrossRef]
- Huang, L.-H.; Zheng, Y.-F.; Lu, Y.-Z.; Song, C.-J.; Wang, Y.-G.; Yu, B.; Liu, H.-M. Synthesis and Biological Evaluation of Novel Steroidal[17,16-d][1,2,4]Triazolo[1,5-a]Pyrimidines. Steroids 2012, 77, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Silvestre, S.M.; Moreira, V.M. Catalytic Oxidative Processes in Steroid Chemistry: Allylic Oxidation, β-Selective Epoxidation, Alcohol Oxidation and Remote Functionalization Reactions. Curr. Org. Chem. 2006, 10, 2227–2257. [Google Scholar] [CrossRef]
- Ten Brink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The Baeyer-Villiger Reaction: New Developments toward Greener Procedures. Chem. Rev. 2004, 104, 4105–4123. [Google Scholar] [CrossRef] [PubMed]
- Meninno, S.; Villano, R.; Lattanzi, A. Magnesium Monoperphthalate (MMPP): A Convenient Oxidant for the Direct Rubottom Oxidation of Malonates, β-Keto Esters, and Amides. Eur. J. Org. Chem. 2021, 2021, 1758–1762. [Google Scholar] [CrossRef]
- Brougham, P.; Cooper, M.; Cummerson, D.; Heaney, H.; Thompson, N. Oxidation Reactions Using Magnesium Monoperphthalate: A Comparison with m-Chloroperoxybenzoic Acid. Synthesis 1987, 1987, 1015–1017. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Vostrikov, N.S. Oxidation with P-(Methoxycarbonyl) Perbenzqic Acid 1. Stereochemistry of Epoxidation of Δ5-Steroids. Bull. Acad. Sci. USSR Div. Chem. Sci. 1978, 27, 387–389. [Google Scholar] [CrossRef]
- Huang, X.; Shen, Q.-K.; Zhang, H.; Li, J.-L.; Tian, Y.Y.-S.; Quan, Z.-S. Design and Synthesis of Novel Dehydroepiandrosterone Analogues as Potent Antiproliferative Agents. Molecules 2018, 23, 2243. [Google Scholar] [CrossRef] [Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Gerdes, J.; Li, L.; Schlueter, C.; Duchrow, M.; Wohlenberg, C.; Gerlach, C.; Stahmer, I.; Kloth, S.; Brandt, E.; Flad, H.D. Immunobiochemical and Molecular Biologic Characterization of the Cell Proliferation-Associated Nuclear Antigen That Is Defined by Monoclonal Antibody Ki-67. Am. J. Pathol. 1991, 138, 867–873. [Google Scholar]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Tan, P.H.; Bay, B.H.; Yip, G.; Selvarajan, S.; Tan, P.; Wu, J.; Lee, C.H.; Li, K. Bin Immunohistochemical Detection of Ki67 in Breast Cancer Correlates with Transcriptional Regulation of Genes Related to Apoptosis and Cell Death. Mod. Pathol. 2005, 18, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.; Giridhar, P.S.; Sandhu, K.; Jader, S.; Sundaresan, V.; Singer, J.; Jenkins, S.; Bradpiece, H.A.; Patel, A. Ki67 in Breast Cancer Patients and Its Correlation with Clinico Pathology Factors. Eur. J. Cancer 2012, 48, S121. [Google Scholar] [CrossRef]
- Chang-Jing, G.Y.; Bae-Li, H.; Faulk, W.P. Propidium Iodide as a Nuclear Marker in Immunofluorescence. Ii. Use with Cellular Identification and Viability Studies. J. Immunol. Methods 1981, 43, 269–275. [Google Scholar] [CrossRef]
- Brana, C.; Benham, C.; Sundstrom, L. A Method for Characterising Cell Death in Vitro by Combining Propidium Iodide Staining with Immunohistochemistry. Brain Res. Protoc. 2002, 10, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Monette, R.; Small, D.L.; Mealing, G.; Morley, P. A Fluorescence Confocal Assay to Assess Neuronal Viability in Brain Slices. Brain Res. Protoc. 1998, 2, 99–108. [Google Scholar] [CrossRef]
- Santos, T.; Ferreira, R.; Quartin, E.; Boto, C.; Saraiva, C.; Bragança, J.; Peça, J.; Rodrigues, C.; Ferreira, L.; Bernardino, L. Blue Light Potentiates Neurogenesis Induced by Retinoic Acid-Loaded Responsive Nanoparticles. Acta Biomater. 2017, 59, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Gobbi, P.; Zamai, L.; Palka, G.; Vitale, M.; Falcieri, E. Morphometric and Functional Study of Apoptotic Cell Chromatin. Cell Death Differ. 1996, 3, 397–405. [Google Scholar]
- Falcieri, E.; Zamai, L.; Santi, S.; Cinti, C.; Gobbi, P.; Bosco, D.; Cataldi, A.; Betts, C.; Vitale, M. The Behaviour of Nuclear Domains in the Course of Apoptosis. Histochemistry 1994, 102, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Varešlija, D.; Tipton, K.F.; Davey, G.P.; McDonald, A.G. 6-Hydroxydopamine: A Far from Simple Neurotoxin. J. Neural Transm. 2020, 127, 213–230. [Google Scholar] [CrossRef]
- Glinka, Y.; Gassen, M.; Youdim, M.B.H. Mechanism of 6-Hydroxydopamine Neurotoxicity. In Advances in Research on Neurodegeneration; Riederer, P., Calne, D.B., Horowski, R., Mizuno, Y., Poewe, W., Youdim, M.B.H., Eds.; Springer: Vienna, Austria, 1997; pp. 55–66. ISBN 978-3-7091-6842-4. [Google Scholar]
- Cagle, B.S.; Sturgeon, M.L.; O’Brien, J.B.; Wilkinson, J.C.; Cornell, R.A.; Roman, D.L.; Doorn, J.A. Stable Expression of the Human Dopamine Transporter in N27 Cells as an in Vitro Model for Dopamine Cell Trafficking and Metabolism. Toxicol. Vitr. 2021, 76, 105210. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-Hydroxydopamine Model of Parkinson’s Disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, E.; Cardounel, A.; Kalimi, M. Pregnenolone Protects Mouse Hippocampal (HT-22) Cells against Glutamate and Amyloid Beta Protein Toxicity. Neurochem. Res. 2001, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bansal, R. Investigations on 16-Arylideno Steroids as a New Class of Neuroprotective Agents for the Treatment of Alzheimer’s and Parkinson’s Diseases. ACS Chem. Neurosci. 2017, 8, 186–200. [Google Scholar] [CrossRef]
- Singh, K.; Nassar, N.; Bachari, A.; Schanknecht, E.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer. Cancers 2021, 13, 4107. [Google Scholar] [CrossRef]
- Tomas-Camardiel, M.; Sanchez-Hidalgo, M.C.; Sanchez del Pino, M.J.; Navarro, A.; Machado, A.; Cano, J. Comparative Study of the Neuroprotective Effect of Dehydroepiandrosterone and 17β-Estradiol against 1-Methyl-4-Phenylpyridium Toxicity on Rat Striatum. Neuroscience 2002, 109, 569–584. [Google Scholar] [CrossRef]
- D’Astous, M.; Morissette, M.; Tanguay, B.; Callier, S.; Di Paolo, T. Dehydroepiandrosterone (DHEA) Such as 17β-Estradiol Prevents MPTP-Induced Dopamine Depletion in Mice. Synapse 2003, 47, 10–14. [Google Scholar] [CrossRef]
- Claessens, F.; Moris, L. The Influence of Steroid Metabolism on CYP17A1 Inhibitor Activity. Nat. Rev. Urol. 2017, 14, 590–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Ar-CHO | Intermediate Product | Final Product | Overall Yield (%) |
|---|---|---|---|
 | 2 | 15 | 69 |
 | 3 | 16 | 30 |
 | 4 | 17 | 86 |
 | 5 | 18 | 58 |
 | 6 | 19 | 76 |
 | 7 | 20 | 86 |
 | 8 | 21 | 79 |
 | 9 | 22 | 85 |
 | 10 | 23 | 65 |
 | 11 | 24 | 58 |
 | 12 | 25 | 69 |
 | 13 | 26 | 86 |
 | 14 | 27 | 65 |
| Compound | NHDF | PNT1A | N27 | LNCaP | PC-3 | MCF-7 |
|---|---|---|---|---|---|---|
| IC50 | IC50 | IC50 | IC50 | IC50 | IC50 | |
| 5-FU | 6.34 | 6.48 | 3.19 | 9.43 | 21.20 | 6.30 |
| 15 | 39.52 | 33.12 | 9.21 | 27.37 | 17.02 | 42.67 |
| 16 | 39.27 | 54.52 | 33.18 | - | 20.06 | - |
| 17 | 32.33 | >100 | >100 | - | - | 20.82 |
| 18 | 29.65 | 29.56 | 12.58 | - | 37.56 | - |
| 19 | 18.69 | 12.75 | 13.32 | 12.83 | 19.03 | 3.47 |
| 20 | 49.96 | 56.63 | 64.46 | - | - | - |
| 21 | 23.44 | 46.33 | 13.16 | - | - | - |
| 22 | 14.95 | 14.41 | 16.77 | 15.02 | 44.46 | 14.46 |
| 23 | 28.34 | 13.27 | 12.74 | 19.29 | 13.97 | 18.68 |
| 24 | 33.43 | 56.46 | 18.92 | - | - | - |
| 25 | 15.98 | 15.33 | 15.36 | 36.88 | 14.52 | 14.52 |
| 26 | 16.62 | 15.58 | 14.34 | 11.34 | 43.76 | 21.82 |
| 27 | 18.40 | 19.47 | 42.35 | 15.80 | - | 56.90 |
| Compound | 5AR | ERα | AR | CYP17A1 | Aromatase |
|---|---|---|---|---|---|
| 15 | −12.5 | −1.5 | −3.3 | −10.2 | −9.8 |
| 16 | −12.4 | −2.3 | 1.8 | −10.2 | −8.0 |
| 17 | −12.7 | −0.7 | 2.4 | −10.5 | −8.2 |
| 18 | −11.8 | −0.6 | −3.9 | −9.8 | −9.7 |
| 19 | −12.7 | −1.5 | −1.7 | −11.0 | −8.3 |
| 20 | −11.9 | −2.1 | −5.8 | −9.8 | −9.6 |
| 21 | −12.4 | −2.1 | −2.4 | −10.3 | −8.8 |
| 22 | −13.7 | 1.2 | 0.0 | −10.3 | −10.2 |
| 23 | −12.7 | −1.5 | 0.6 | −10.5 | −8.5 |
| 24 | −13.0 | −1.4 | −3.1 | −10.8 | −8.8 |
| 25 | −12.5 | −0.3 | 0.7 | −10.5 | −8.0 |
| 26 | −11.9 | −1.5 | −0.5 | −10.1 | −8.3 |
| 27 | −12.4 | −2.3 | −1.6 | −10.3 | −8.8 |
| NADP-DHF | −8.4 | - | - | - | - |
| Finasteride | −11.9 | - | - | - | - |
| Estradiol | - | −10.4 | - | - | - |
| DHT | - | - | −11.2 | - | - |
| Abiraterone | - | - | - | −10.2 | - |
| Androstenedione | - | - | - | - | −10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, V.; Marques, M.; Esteves, M.; Serra-Almeida, C.; Alves, G.; Almeida, P.; Bernardino, L.; Silvestre, S. Synthesis, In Vitro Biological Evaluation of Antiproliferative and Neuroprotective Effects and In Silico Studies of Novel 16E-Arylidene-5α,6α-epoxyepiandrosterone Derivatives. Biomedicines 2023, 11, 812. https://doi.org/10.3390/biomedicines11030812
Brito V, Marques M, Esteves M, Serra-Almeida C, Alves G, Almeida P, Bernardino L, Silvestre S. Synthesis, In Vitro Biological Evaluation of Antiproliferative and Neuroprotective Effects and In Silico Studies of Novel 16E-Arylidene-5α,6α-epoxyepiandrosterone Derivatives. Biomedicines. 2023; 11(3):812. https://doi.org/10.3390/biomedicines11030812
Chicago/Turabian StyleBrito, Vanessa, Mariana Marques, Marta Esteves, Catarina Serra-Almeida, Gilberto Alves, Paulo Almeida, Liliana Bernardino, and Samuel Silvestre. 2023. "Synthesis, In Vitro Biological Evaluation of Antiproliferative and Neuroprotective Effects and In Silico Studies of Novel 16E-Arylidene-5α,6α-epoxyepiandrosterone Derivatives" Biomedicines 11, no. 3: 812. https://doi.org/10.3390/biomedicines11030812