Bacterial-Specific Induction of Inflammatory Cytokines Significantly Decreases upon Dual Species Infections of Implant Materials with Periodontal Pathogens in a Mouse Model
Abstract
:1. Introduction
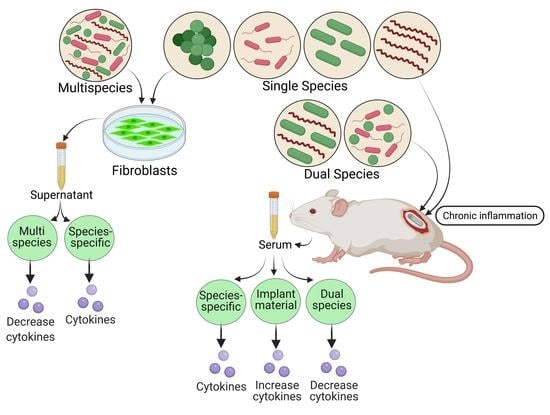
2. Materials and Methods
2.1. Bacterial Cultivation
2.2. Co-Cultivation of Murine Fibroblasts with Bacteria
2.3. Preparation of Titanium Implants
2.4. Subcutaneous Implantations and Infections in Small Animal Model
2.5. Measurement of Cytokines in Serum
2.6. Statistical Analysis
3. Results
3.1. Cytokines Expression in Murine Fibroblasts
3.2. Systemic Analysis of Serum Interleukin
3.3. Systemic Analysis of Serum Chemokines
3.4. Systemic Analysis of Growth Factors and Cellular Regulators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannobile, W.; Lang, N. Are dental implants a panacea or should we better strive to save teeth. J. Dent. Res. 2016, 95, 5–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. Npj Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Kabir, L.; Stiesch, M.; Grischke, J. The effect of keratinized mucosa on the severity of peri-implant mucositis differs between periodontally healthy subjects and the general population: A cross-sectional study. Clin. Oral Investig. 2021, 25, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Bremer, F.; Grade, S.; Kohorst, P.; Stiesch, M. In vivo biofilm formation on different dental ceramics. Quintessence Int. 2011, 42, 565–574. [Google Scholar]
- Jakobi, M.L.; Stumpp, S.N.; Stiesch, M.; Eberhard, J.; Heuer, W. The Peri-Implant and Periodontal Microbiota in Patients with and without Clinical Signs of Inflammation. Dent. J. 2015, 3, 24–42. [Google Scholar] [CrossRef] [Green Version]
- Ingendoh-Tsakmakidis, A.; Mikolai, C.; Winkel, A.; Szafrański, S.P.; Falk, C.S.; Rossi, A.; Walles, H.; Stiesch, M. Commensal and pathogenic biofilms differently modulate peri-implant oral mucosa in an organotypic model. Cell. Microbiol. 2019, 21, e13078. [Google Scholar] [CrossRef] [Green Version]
- Vantucci, C.E.; Ahn, H.; Schenker, M.L.; Pradhan, P.; Wood, L.B.; Guldberg, R.E.; Roy, K.; Willett, N.J. Development of Systemic Immune Dysregulation in a Rat Trauma Model with Biomaterial-Associated Infection. Biomaterials 2021, 264, 120405. [Google Scholar] [CrossRef]
- Torrado, E.; Cooper, A.M. Cytokines in the balance of protection and pathology during mycobacterial infections. Adv. Exp. Med. Biol. 2013, 783, 121–140. [Google Scholar]
- Rahim, M.I.; Babbar, A.; Lienenklaus, S.; Pils, M.C.; Rohde, M. Degradable magnesium implant-associated infections by bacterial biofilms induce robust localized and systemic inflammatory reactions in a mouse model. Biomed. Mater. 2017, 12, 055006. [Google Scholar] [CrossRef] [Green Version]
- Rochford, E.T.J.; Sabaté Brescó, M.; Zeiter, S.; Kluge, K.; Poulsson, A.; Ziegler, M.; Richards, R.G.; O’Mahony, L.; Moriarty, T.F. Monitoring immune responses in a mouse model of fracture fixation with and without Staphylococcus aureus osteomyelitis. Bone 2016, 83, 82–92. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H.F. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J., Jr.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [Green Version]
- Alves, L.A.; de Carli, T.R.; Harth-Chu, E.N.; Mariano, F.S.; Höfling, J.F.; Stipp, R.N.; Mattos-Graner, R.O. Oral streptococci show diversity in resistance to complement immunity. J. Med. Microbiol. 2019, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Bullon, P.; Fioroni, M.; Goteri, G.; Rubini, C.; Battino, M. Immunohistochemical analysis of soft tissues in implants with healthy and peri-implantitis condition, and aggressive periodontitis. Clin. Oral Implant. Res. 2004, 15, 553–559. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef] [Green Version]
- Radaelli, K.; Alberti, A.; Corbella, S.; Francetti, L. The Impact of Peri-Implantitis on Systemic Diseases and Conditions: A Review of the Literature. Int. J. Dent. 2021, 2021, 5536566. [Google Scholar] [CrossRef]
- Rahim, M.I.; Winkel, A.; Lienenklaus, S.; Stumpp, N.S.; Szafrański, S.P.; Kommerein, N.; Willbold, E.; Reifenrath, J.; Mueller, P.P.; Eisenburger, M.; et al. Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions. Microorganisms 2020, 8, 1624. [Google Scholar] [CrossRef]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Kommerein, N.; Stumpp, S.N.; Müsken, M.; Ehlert, N.; Winkel, A.; Häussler, S.; Behrens, P.; Buettner, F.F.R.; Stiesch, M. An oral multispecies biofilm model for high content screening applications. PLoS ONE 2017, 12, e0173973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.C.S.; Siboo, R.; Keng, T.; Psarra, N.; Hurley, R.; Cheng, S.-L.; Iugovaz, I. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and Identification of New Spirochete Isolates from Periodontal Pockets. Int. J. Syst. Evol. Microbiol. 1993, 43, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahim, M.I.; Tavares, A.; Evertz, F.; Kieke, M.; Seitz, J.-M.; Eifler, R.; Weizbauer, A.; Willbold, E.; Maier, H.J.; Glasmacher, B.; et al. Phosphate conversion coating reduces the degradation rate and suppresses side effects of metallic magnesium implants in an animal model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and characterization of an oral multispecies biofilm implant flow chamber model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef] [Green Version]
- Moser, B.; Willimann, K. Chemokines: Role in inflammation and immune surveillance. Ann. Rheum. Dis. 2004, 63, ii84–ii89. [Google Scholar] [CrossRef]
- Laine, M.L.; Leonhardt, Å.; Roos-Jansåker, A.-M.; Peña, A.S.; Van Winkelhoff, A.J.; Winkel, E.G.; Renvert, S. IL-1RN gene polymorphism is associated with peri-implantitis. Clin. Oral Implant. Res. 2006, 17, 380–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Jinbu, Y.; Itoh, H.; Kusama, M. Increased IL-6 Levels in Peri-Implant Crevicular Fluid Correlate with Peri-Implantitis. Oral Med. Pathol. 2005, 10, 95–99. [Google Scholar] [CrossRef]
- Fonseca, F.J.P.O.; Junior, M.M.; Lourenço, E.J.V.; de Moraes Teles, D.; Figueredo, C.M. Cytokines expression in saliva and peri-implant crevicular fluid of patients with peri-implant disease. Clin. Oral Implant. Res. 2014, 25, e68–e72. [Google Scholar] [CrossRef]
- Liskmann, S.; Vihalemm, T.; Salum, O.; Zilmer, K.; Fischer, K.; Zilmer, M. Correlations Between Clinical Parameters and lnterleukin-6 and lnterleukin-10 Levels in Saliva from Totally Edentulous Patients with Peri-implant Disease. Int. J. Oral Maxillofac. Implant. 2006, 21, 543–550. [Google Scholar]
- Faot, F.; Nascimento, G.G.; Bielemann, A.M.; Campão, T.D.; Leite, F.R.M.; Quirynen, M. Can Peri-Implant Crevicular Fluid Assist in the Diagnosis of Peri-Implantitis? A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 631–645. [Google Scholar] [CrossRef]
- Zani, S.R.; Moss, K.; Shibli, J.A.; Teixeira, E.R.; de Oliveira Mairink, R.; Onuma, T.; Feres, M.; Teles, R.P. Peri-implant crevicular fluid biomarkers as discriminants of peri-implant health and disease. J. Clin. Periodontol. 2016, 43, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Kobayashi, Y.; Yamasaki, S.; Kawakami, A.; Eguchi, K.; Sasaki, H.; Sakai, H. Protein Expression and Functional Difference of Membrane-Bound and Soluble Receptor Activator of NF-κB Ligand: Modulation of the Expression by Osteotropic Factors and Cytokines. Biochem. Biophys. Res. Commun. 2000, 275, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Severino, V.O.; Napimoga, M.H.; de Lima Pereira, S.A. Expression of IL-6, IL-10, IL-17 and IL-8 in the peri-implant crevicular fluid of patients with peri-implantitis. Arch. Oral Biol. 2011, 56, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Götz, W.; Deschner, J.; Jäger, A.; Nogueira, A.V.B. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues—In vitro and in vivo studies. Clin. Oral Investig. 2020, 24, 3661–3670. [Google Scholar] [CrossRef] [Green Version]
- Gemmell, E.; Carter, C.L.; Seymour, G.J. Chemokines in human periodontal disease tissues. Clin. Exp. Immunol. 2001, 125, 134–141. [Google Scholar] [CrossRef]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 alpha)/CCL3: As a Biomarker. Gen. Methods Biomark. Res. Appl. 2015, 27, 223–249. [Google Scholar] [CrossRef]
- De Wilde, E.A.W.J.; Jimbo, R.; Wennerberg, A.; Naito, Y.; Coucke, P.; Bryington, M.S.; Vandeweghe, S.; De Bruyn, H. The Soft Tissue Immunologic Response to Hydroxyapatite-Coated Transmucosal Implant Surfaces: A Study in Humans. Clin. Implant. Dent. Relat. Res. 2015, 17, e65–e74. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Vissink, A.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Liefers, S.C.; Kroese, F.G.M.; Raghoebar, G.M. Biomarker levels in peri-implant crevicular fluid of healthy implants, untreated and non-surgically treated implants with peri-implantitis. J. Clin. Periodontol. 2021, 48, 590–601. [Google Scholar] [CrossRef]
- Petković, A.B.; Matić, S.M.; Stamatović, N.V.; Vojvodić, D.V.; Todorović, T.M.; Lazić, Z.R.; Kozomara, R.J. Proinflammatory cytokines (IL-1β and TNF-α) and chemokines (IL-8 and MIP-1α) as markers of peri-implant tissue condition. Int. J. Oral Maxillofac. Surg. 2010, 39, 478–485. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Renvert, S.; Roos-Jansåker, A.-M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lienenklaus, S.; Cornitescu, M.; Zietara, N.; Łyszkiewicz, M.; Gekara, N.; Jabłónska, J.; Edenhofer, F.; Rajewsky, K.; Bruder, D.; Hafner, M.; et al. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J. Immunol. 2009, 183, 3229–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingendoh-Tsakmakidis, A.; Eberhard, J.; Falk, C.S.; Stiesch, M.; Winkel, A. In Vitro Effects of Streptococcus oralis Biofilm on Peri-Implant Soft Tissue Cells. Cells 2020, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Belton, C.M.; Reife, R.A.; Lamont, R.J. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 1998, 66, 1660–1665. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczyk-Pawlinska, J.; Travis, J.; Potempa, J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: Implications for pathogenicity of periodontal disease. FEBS Lett. 1998, 440, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Sandros, J.; Karlsson, C.; Lappin, D.F.; Madianos, P.N.; Kinane, D.F.; Papapanou, P.N. Cytokine Responses of Oral Epithelial Cells to Porphyromonas gingivalis Infection. J. Dent. Res. 2000, 79, 1808–1814. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kita, M.; Oseko, F.; Nakamura, T.; Imanishi, J.; Kanamura, N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J. Periodontal Res. 2006, 41, 554–559. [Google Scholar] [CrossRef]
- Tan, K.H.; Seers, C.A.; Dashper, S.G.; Mitchell, H.L.; Pyke, J.S.; Meuric, V.; Slakeski, N.; Cleal, S.M.; Chambers, J.L.; McConville, M.J.; et al. Porphyromonas gingivalis and Treponema denticola Exhibit Metabolic Symbioses. PLoS Pathog. 2014, 10, e1003955. [Google Scholar] [CrossRef]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter actinomycetemcomitans: Virulence of its leukotoxin and association with aggressive periodontitis. Virulence 2015, 6, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Palm, E.; Khalaf, H.; Bengtsson, T. Porphyromonas gingivalis downregulates the immune response of fibroblasts. BMC Microbiol. 2013, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Abdi, K.; Chen, T.; Klein, B.A.; Tai, A.K.; Coursen, J.; Liu, X.; Skinner, J.; Periasamy, S.; Choi, Y.; Kessler, B.M.; et al. Mechanisms by which Porphyromonas gingivalis evades innate immunity. PLoS ONE 2017, 12, e0182164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, C.S.; Steffen, M.J.; Ebersole, J.L. Cytokine responses to treponema pectinovorum and treponema denticola in human gingival fibroblasts. Infect. Immun. 2000, 68, 5284–5292. [Google Scholar] [CrossRef] [Green Version]
- Asai, Y.; Jinno, T.; Ogawa, T. Oral treponemes and their outer membrane extracts activate human gingival epithelial cells through toll-like receptor. Infect. Immun. 2003, 71, 717–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelens, J.J.; Zaat, S.A.J.; Murk, J.L.; Weening, J.J.; van der Poll, T.; Dankert, J. Enhanced Susceptibility to Subcutaneous Abscess Formation and Persistent Infection around Catheters Is Associated with Sustained Interleukin-1β Levels. Infect. Immun. 2000, 68, 1692–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelens, J.J.; van der Poll, T.; Zaat, S.A.J.; Murk, J.L.A.N.; Weening, J.J.; Dankert, J. Interleukin-1 Receptor Type I Gene-Deficient Mice Are Less Susceptible to Staphylococcus epidermidis Biomaterial-Associated Infection than Are Wild-Type Mice. Infect. Immun. 2000, 68, 6924–6931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, M.A.; Simmons, R.L.; Kaplan, S.S. TNF and IL-1 generation by human monocytes in response to biomaterials. J. Biomed. Mater. Res. 1992, 26, 851–859. [Google Scholar] [CrossRef]
- Nilsdotter-Augustinsson, Å.; Briheim, G.; Herder, A.; Ljunghusen, O.; Wahlström, O.; öhman, L. Inflammatory response in 85 patients with loosened hip prostheses: A prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007, 78, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Skadiņš, I.; Kroiča, J.; Salma, I.; Reinis, A.; Sokolova, M.; Rostoka, D. The Level of Inflammatory Cytokines and Antimicrobial Peptides after Composite Material Implantation and Contamination with Bacterial Culture. Key Eng. Mater. 2017, 721, 245–250. [Google Scholar] [CrossRef]
- Andrukhov, O.; Ulm, C.; Reischl, H.; Nguyen, P.Q.; Matejka, M.; Rausch-Fan, X. Serum Cytokine Levels in Periodontitis Patients in Relation to the Bacterial Load. J. Periodontol. 2011, 82, 885–892. [Google Scholar] [CrossRef]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6, 33123. [Google Scholar] [CrossRef] [Green Version]
- Ritzman, A.M.; Hughes-Hanks, J.M.; Blaho, V.A.; Wax, L.E.; Mitchell, W.J.; Brown, C.R. The chemokine receptor CXCR2 ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect. Immun. 2010, 78, 4593–4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, C.J.; Martin, T.R.; Frevert, C.W.; Quan, J.M.; Wong, V.A.; Mongovin, S.M.; Hagen, T.R.; Steinberg, K.P.; Goodman, R.B. Expression and Function of the Chemokine Receptors CXCR1 and CXCR2 in Sepsis. J. Immunol. 1999, 162, 2341. [Google Scholar] [PubMed]
- Jin, L.; Batra, S.; Douda, D.N.; Palaniyar, N.; Jeyaseelan, S. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J. Immunol. 2014, 193, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Darveau, R.P.; Pham, T.T.; Lemley, K.; Reife, R.A.; Bainbridge, B.W.; Coats, S.R.; Howald, W.N.; Way, S.S.; Hajjar, A.M. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 2004, 72, 5041–5051. [Google Scholar] [CrossRef] [Green Version]
- Bostanci, N.; Allaker, R.P.; Belibasakis, G.N.; Rangarajan, M.; Curtis, M.A.; Hughes, F.J.; McKay, I.J. Porphyromonas gingivalis antagonises Campylobacter rectus induced cytokine production by human monocytes. Cytokine 2007, 39, 147–156. [Google Scholar] [CrossRef]
- Bostanci, N.; Allaker, R.; Johansson, U.; Rangarajan, M.; Curtis, M.A.; Hughes, F.J.; McKay, I.J. Interleukin-1α stimulation in monocytes by periodontal bacteria: Antagonistic effects of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2007, 22, 52–60. [Google Scholar] [CrossRef]
- Murray, D.A.; Wilton, J.M.A. Lipopolysaccharide from the periodontal pathogen Porphyromonas gingivalis prevents apoptosis of HL60-derived neutrophils in vitro. Infect. Immun. 2003, 71, 7232–7235. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G.; Martin, M.; Schifferle, R.E.; Genco, R.J. Counteracting interactions between lipopolysaccharide molecules with differential activation of toll-like receptors. Infect. Immun. 2002, 70, 6658–6664. [Google Scholar] [CrossRef] [Green Version]
- Bodet, C.; Chandad, F.; Grenier, D. Inflammatory responses of a macrophage/epithelial cell co-culture model to mono and mixed infections with Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. Microbes Infect. 2006, 8, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Krauss, J.L.; Liang, S.; McIntosh, M.L.; Lambris, J.D. Pathogenic microbes and community service through manipulation of innate immunity. Adv. Exp. Med. Biol. 2012, 946, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Cytokines | Implant vs. So-Implant | Implant vs. Aa-Implant | Implant vs. Pg-Implant | Implant vs. Td-Implant | Implant vs. SoPg-Implant | Implant vs. AaTd-Implant | Importance in Peri-Implantitis |
|---|---|---|---|---|---|---|---|
| IL-1α | ↓*** | High expression in the manifestation of peri-implantitis [26] | |||||
| IL-2 | ↓# | ↑* | ↓# | ↓*** | ↓*** | Higher levels in peri-implantitis [27] | |
| IL-5 | ↓* | ↓*** | ↓*** | Expression in peri-implantitis was not significantly higher compared to healthy patients [28] | |||
| IL-6 | ↓** | The concentration remains higher in patients with peri- implantitis than in healthy implants [29] | |||||
| IL-9 | ↓** | High expression not reported in peri-implantitis | |||||
| IL-12 (p40) | ↓*** | ↓*** | High expression in peri-implantitis [30] | ||||
| IL-12 (p70) | ↓# | ↓# | ↓# | ↓** | ↓# | High expression in peri-implantitis [30,31] | |
| IL-13 | ↓# | ↓*** | ↓** | Antiresorptive agent to suppress osteoclastogenesis [32] | |||
| IL-17 | Higher levels in peri-implantitis [33] | ||||||
| CXCL1/KC | ↑** | ↑# | Higher levels are found in periodontitis compared to healthy sites [34] | ||||
| CCL5/RANTES | ↓** | ↓# | ↓** | Pro-osteogenic: associated with macrophage transition from (M1) to (M2) phase [35] | |||
| CCL-3/MIP1α | ↑# | ↓# | ↓# | Inflammation and bone resorption in periodontitis [36]; no statistically significant role reported thus far in peri-implantitis [37] | |||
| IFN-γ | ↑# | ↓** | IFN-γ considered as antiresorptive agents by suppressing osteoclastogenesis | ||||
| G-CSF | ↑# | ↓# | ↓# | No difference reported thus far in patients with peri-implantitis compared to patients with healthy implants [38] | |||
| TNF-α | ↓# | Pro-inflammatory, promotes alveolar bone loss, augments production in peri-implantitis [39] | |||||
| CCL2/MCP-1 | ↓# | ↓*** | ↓*** | Higher levels are found in periodontitis [34]; macrophages produce to promote fibrosis [40] | |||
| Upregulated | 1 | 4 | 0 | 1 | 0 | 0 | |
| Downregulated | 2 | 0 | 5 | 2 | 12 | 11 |
| Cytokines. | So-Implant vs. So | Aa-Implant vs. Aa | Pg-Implant vs. Pg | Td-Implant vs. Td | SoPg-Implant vs. SoPg | AaTd-Implant vs. AaTd |
|---|---|---|---|---|---|---|
| IL-1α | ↑* | ↑*** | ↓# | |||
| IL-2 | ↓# | ↑# | ||||
| IL-3 | ↑# | |||||
| IL-5 | ↑* | |||||
| IL-6 | ↑** | ↓# | ↑*** | ↓# | ||
| IL-9 | ↓* | ↑** | ||||
| IL-12(p40) | ↑# | ↓** | ↓*** | ↓# | ||
| IL-12(p70) | ↓# | |||||
| IL-13 | ↑# | |||||
| CXCL1/KC | ↑# | ↑# | ||||
| CCL5/RANTES | ↑* | ↓# | ↓** | |||
| CCL-3/MIP1α | ↑* | ↑# | ↓# | |||
| CCL11 | ↑# | ↑# | ||||
| IFN-γ | ↓# | ↓* | ||||
| G-CSF | ↓# | |||||
| CCL2/MCP-1 | ↑# | ↑* | ||||
| Upregulated | 6 | 5 | 2 | 5 | 0 | 1 |
| Downregulated | 1 | 0 | 4 | 0 | 6 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, M.I.; Winkel, A.; Ingendoh-Tsakmakidis, A.; Lienenklaus, S.; Falk, C.S.; Eisenburger, M.; Stiesch, M. Bacterial-Specific Induction of Inflammatory Cytokines Significantly Decreases upon Dual Species Infections of Implant Materials with Periodontal Pathogens in a Mouse Model. Biomedicines 2022, 10, 286. https://doi.org/10.3390/biomedicines10020286
Rahim MI, Winkel A, Ingendoh-Tsakmakidis A, Lienenklaus S, Falk CS, Eisenburger M, Stiesch M. Bacterial-Specific Induction of Inflammatory Cytokines Significantly Decreases upon Dual Species Infections of Implant Materials with Periodontal Pathogens in a Mouse Model. Biomedicines. 2022; 10(2):286. https://doi.org/10.3390/biomedicines10020286
Chicago/Turabian StyleRahim, Muhammad Imran, Andreas Winkel, Alexandra Ingendoh-Tsakmakidis, Stefan Lienenklaus, Christine S. Falk, Michael Eisenburger, and Meike Stiesch. 2022. "Bacterial-Specific Induction of Inflammatory Cytokines Significantly Decreases upon Dual Species Infections of Implant Materials with Periodontal Pathogens in a Mouse Model" Biomedicines 10, no. 2: 286. https://doi.org/10.3390/biomedicines10020286