Architectures and Mechanisms of Perylene Diimide-Based Optical Chemosensors for pH Probing
Abstract
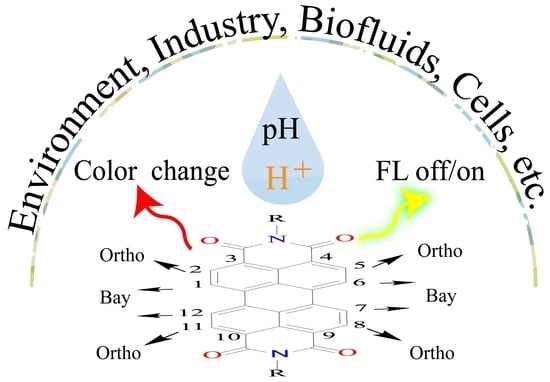
:1. Introduction
2. Colorimetric Chemosensors
2.1. Hydrochromism for pH and Humidity Sensing
2.2. Synergistic CO2 and pH Sensing
3. Fluorescent Chemosensors
3.1. pH Sensing Based on Photoinduced Electron Transfer (PET) Mechanism
3.2. pH Sensing Based on Supramolecular (De)Aggregation Mechanism
3.3. pH Sensing Based on Fluorescence Resonance Energy Transfer (FRET) Mechanism
3.4. pH Sensing Based on Tunable Lateral Dimensions of 1D Nanostructures
3.5. pH Sensing Based on Volume Phase Transition of Unimolecular Micelle
3.6. pH Imaging in Cells
3.6.1. Dendritic PDIs for Live-Cell Imaging
3.6.2. Fluorescence Lifetime Probing
4. Conclusions and Perspectives
4.1. pH-Resistant Fluorescence Probes
4.2. pH Response within Strong Acidic or Basic System
4.3. pH Response with PDI-Involved Composites
4.4. pH-Mediated Detection of Other Analytes
4.5. Integrating pH Probe into Devices
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Slattum, P.; Wang, C.Y.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Tao, D.D.; Zhang, Q.; Zhang, P.; Guo, D.P.; Jiang, Y.B. Supramolecular aggregates as sensory ensembles. Chem. Commun. 2016, 52, 12929–12939. [Google Scholar] [CrossRef]
- Chen, S.; Xue, Z.X.; Gao, N.; Yang, X.M.; Zang, L. Perylene diimide-based fluorescent and colorimetric sensors for environmental detection. Sensors 2020, 20, 917. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S.J. Recent advances in perylene diimide-based active materials in electrical mode gas sensing. Chemosensors 2021, 9, 30. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P.; Kaur, N.; Mittal, L.S.; Kumar, K. Perylene diimides: Will they flourish as reaction-based probes? Anal. Methods 2020, 12, 3560–3574. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.F.; Liao, C.L.; Tian, Q.Y.; Wang, C.Y.; Chen, S.; Zang, L. Perylene imide-based optical chemosensors for vapor detection. Chemosensors 2021, 9, 1. [Google Scholar] [CrossRef]
- Zhou, W.W.; Liu, G.; Yang, B.; Ji, Q.Y.; Xiang, W.M.; He, H.; Xu, Z.; Qi, C.D.; Li, S.; Yang, S.G.; et al. Review on application of perylene diimide (PDI)-based materials in environment: Pollutant detection and degradation. Sci. Total Environ. 2021, 780, 146483. [Google Scholar] [CrossRef]
- Singh, P.; Hirsch, A.; Kumar, S. Perylene diimide-based chemosensors emerging in recent years: From design to sensing. TrAC Trends Anal. Chem. 2021, 138, 116237. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical sensing and imaging of pH values: Spectroscopies, materials, and applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef]
- Aigner, D.; Borisov, S.M.; Klimant, I. New fluorescent perylene bisimide indicators—A platform for broadband pH optodes. Anal. Bioanal. Chem. 2011, 400, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Würthner, F. Halochromic and hydrochromic squaric acid functionalized perylene bisimide. Chem. Comm. 2015, 51, 7661–7664. [Google Scholar] [CrossRef]
- Pfeifer, D.; Klimant, I.; Borisov, S.M. Ultrabright red-emitting photostable perylene bisimide dyes: New indicators for ratiometric sensing of high pH or carbon dioxide. Chem. Eur. J. 2018, 24, 10711–10720. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Li, P.; Liu, Y.L.; Liang, X.M.; Fu, Y.; Ye, F. A dual-mode colorimetric/fluorescent probe based on perylene: Response to acidic pH values. J. Taiwan Inst. Chem. Eng. 2021, 129, 97–103. [Google Scholar] [CrossRef]
- Zang, L.; Liu, R.C.; Holman, M.W.; Nguyen, K.T.; Adams, D.M. A single-molecule probe based on intramolecular electron transfer. J. Am. Chem. Soc. 2002, 124, 10640–10641. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Liang, X.M.; Wu, N.; Li, P.; Chai, Q.; Fu, Y. A new perylene-based fluorescent pH chemosensor for strongly acidic condition. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 359–364. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, S.Y.; Li, F.H.; Han, D.X.; Zhang, Q.X.; Niu, L. pH responding reversible supramolecular self-assembly of water-soluble amino-imidazole-armed perylene diimide dye for biological applications. RSC Adv. 2015, 5, 2207–2212. [Google Scholar] [CrossRef]
- Li, S.Y.; Long, T.; Wang, Y.; Yang, X.G. Self-assembly, protonation-dependent morphology, and photophysical properties of perylene bisimide with tertiary amine groups. Dyes Pigm. 2020, 173, 107896. [Google Scholar] [CrossRef]
- Zhang, X.; Rehm, S.; Safont-Sempere, M.M.; Würthner, F. Vesicular perylene dye nanocapsules as supramolecular fluorescent pH sensor systems. Nature Chem. 2009, 1, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Pandeeswar, M.; Govindaraju, T. Engineering molecular self-assembly of perylene diimide through pH-responsive chiroptical switching. Mol. Syst. Des. Eng. 2016, 1, 202–207. [Google Scholar] [CrossRef]
- You, S.S.; Cai, Q.; Müllen, K.; Yang, W.T.; Yin, M.Z. pH-sensitive unimolecular fluorescent polymeric micelles: From volume phase transition to optical response. Chem. Commun. 2014, 50, 823–825. [Google Scholar] [CrossRef]
- Gao, B.X.; Li, H.X.; Liu, H.M.; Zhang, L.C.; Bai, Q.Q.; Ba, X.W. Water-soluble and fluorescent dendritic perylene bisimides for live-cell imaging. Chem. Commun. 2011, 47, 3894–3896. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, F.; Zhang, J.; Jiang, T.; Li, X.; Wu, J.; Ren, H. A water-soluble fluorescent pH probe based on perylene dyes and its application to cell imaging. Luminescence 2016, 31, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dyes Pigm. 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Aigner, D.; Dmitriev, R.I.; Borisov, S.M.; Papkovsky, D.B.; Klimant, I. pH-sensitive perylene bisimide probes for live cell fluorescence lifetime imaging. J. Mater. Chem. B 2014, 2, 6792–6801. [Google Scholar] [CrossRef]
- Pacheco-Linan, P.J.; Moral, M.; Nueda, M.L.; Cruz-Sanchez, R.; Fernandez-Sainz, J.; Garzon-Ruiz, A.; Bravo, I.; Melguizo, M.; Laborda, J.; Albaladejo, J. Study on the pH dependence of the photophysical properties of a functionalized perylene bisimide and its potential applications as a fluorescence lifetime based pH probe. J. Phys. Chem. C 2017, 121, 24786–24797. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Sakr, A.R.; Bojinov, V.B. Design and synthesis of novel fluorescence sensing perylene diimides based on photoinducedelectron transfer. Dyes Pigm. 2011, 91, 332–339. [Google Scholar] [CrossRef]
- Daffy, L.M.; Silva, A.P.D.; Gunaratne, H.Q.N.; Huber, C.; Lynch, P.L.M.; Werner, T.; Wolfbeis, O.S. Arenedicarboximide building blocks for fluorescent photoinduced electron transfer pH sensors applicable with different media and communication wavelengths. Chem. Eur. J. 1998, 4, 1810–1815. [Google Scholar] [CrossRef]
- Golshan, M.; Rostami-Tapeh-Esmail, E.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. A review on synthesis, photophysical properties, and applications of dendrimers with perylene core. Eur. Polym. J. 2020, 137, 109933. [Google Scholar] [CrossRef]
- Sakr, A.R.; Georgiev, N.I.; Bojinov, V.B. Design, synthesis, and biological activity of perylene tetracarboxydiimide dendrimer. Synth. Commun. 2022, 52, 2171–2177. [Google Scholar] [CrossRef]
- Wu, J.H.; Peng, M.; Mu, M.X.; Li, J.; Yin, M.Z. Perylene diimide supramolecular aggregates: Constructions and sensing applications. Supramol. Mater. 2023, 2, 100031. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.F.; Han, Y.F. A perylene diimide-based fluorescent probe for the selective detection of hypochlorite in living cells. Mater. Chem. Front. 2022, 6, 2266–2273. [Google Scholar] [CrossRef]
- Ma, L.; Gao, W.J.; Han, X.; Qu, F.L.; Xia, L.; Kong, R.M. A label-free and fluorescence turn-on assay for sensitive detection of hyaluronidase based on hyaluronan-induced perylene self-assembly. New. J. Chem. 2019, 43, 3383–3389. [Google Scholar] [CrossRef]
- Kar, M.; Anas, M.; Banerjee, P.; Singh, A.; Sen, P.; Mandal, T.K. Amphiphilic perylene bisimide–polymer conjugates by cysteine-based orthogonal strategy: Vesicular aggregation, DNA binding, and cell imaging. ACS Appl. Polym. Mater. 2022, 4, 3697–3710. [Google Scholar] [CrossRef]
- Zhao, Z.N.; Xu, N.; Wang, Y.; Ling, G.X.; Zhang, P. Perylene diimide-based treatment and diagnosis of diseases. J. Mater. Chem. B 2021, 9, 8937–8950. [Google Scholar] [CrossRef] [PubMed]
- Padghan, S.D.; Chung, M.C.; Zhang, Q.S.; Lin, W.C.; Chen, K.Y. 1,6,7-Trisubstituted perylene bisimides with tunable optical properties for colorimetric and “turn-on” fluorescence detection of HCl. Dyes Pigm. 2022, 202, 110303. [Google Scholar] [CrossRef]
- Hariharan, P.S.; Pitchaimani, J.; Madhu, V.; Anthony, S.P. Perylene diimide based fluorescent dyes for selective sensing of nitroaromatic compounds: Selective sensing in aqueous medium across wide pH range. J. Fluoresc. 2016, 26, 395–401. [Google Scholar] [CrossRef]
- Zhang, F.X.; Dong, W.Y.; Ma, Y.S.; Jiang, T.Y.; Liu, B.; Li, X.M.; Shao, Y.Y.; Wu, J.S. Fluorescent pH probes for alkaline pH range based on perylene tetra-(alkoxycarbonyl) derivatives. Arab. J. Chem. 2020, 13, 5900–5910. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, Z.R.; Jin, L.L.; Ye, B.L.; Zhou, J.; Sun, Z.Q.; Yang, H.; Hong, L.J.; Huang, F.H.; Wang, W.L.; et al. An NIR discrete metallacycle constructed from perylene bisimide and tetraphenylethylene fluorophores for imaging-guided cancer radio-chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, T.H.; Peng, H.N.; Fang, Y. Advances in molecular design and photophysical engineering of perylene bisimide-containing polyads and multichromophores for film-based fluorescent sensors. J. Phys. Chem. B 2023, 127, 828–837. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chou, Y.T.; Su, B.K.; Wu, C.C.; Wang, C.H.; Chang, K.H.; Annie Ho, J.; Chou, P.T. Comprehensive thione-derived perylene diimides and their bio-conjugation for simultaneous imaging, tracking, and targeted photodynamic therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef]
- Kar, M.; Anas, M.; Singh, A.; Basak, A.; Sen, P.; Mandal, T.K. Ion-/thermo-responsive fluorescent perylene-poly(ionic liquid) conjugates: One-pot microwave synthesis, self-aggregation and biological applications. Eur. Polym. J. 2022, 179, 111561. [Google Scholar] [CrossRef]
- Li, C.W.; Gao, Y.; Huang, R.; Fang, L.; Sun, Y.Y.; Yang, Y.L.; Gou, S.H.; Zhao, J. An effective supramolecular approach to boost the photodynamic therapy efficacy of a near-infrared activating perylene diimide-based photosensitizer. ACS Mater. Lett. 2022, 4, 657–664. [Google Scholar] [CrossRef]
- Ali, T.H.; Mandal, A.M.; Alhasan, A.; Dehaen, W. Surface fabrication of magnetic core-shell silica nanoparticles with perylene diimide as a fluorescent dye for nucleic acid visualization. J. Mol. Liq. 2022, 359, 119345. [Google Scholar]
- Ding, J.; Sun, M.M.; Liu, J.M.; Liu, X.Q.; Hou, W.L.; Liu, L.; Zhang, H.Q. Colorimetric switching and sensing of CO2 based on reversible proton movement between the two heavy atoms in low barrier hydrogen bond in PDI radical anion/b-PEI hydrogen bonding complex. Sens. Actuators B Chem. 2022, 372, 132685. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.P.; Wang, H.L.; Zhu, Y.H.; Sun, M.M.; Wang, Q.; Zhang, H.Q. Detecting metal ions by the color change in perylene diimide radical anion/b-PEI complex. Dyes Pigm. 2023, 210, 110942. [Google Scholar] [CrossRef]
- Kwon, N.Y.; Kim, Y.; Kataria, M.; Park, S.H.; Cho, S.; Harit, A.K.; Woo, H.Y.; Cho, M.J.; Park, M.; Choi, D.H. Donor-σ-acceptor dyad-based polymers for portable sensors: Controlling photoinduced electron transfer via tuning the frontier molecular orbital energies of acceptors. Macromolecules 2022, 55, 1609–1619. [Google Scholar] [CrossRef]
- Ding, S.; Zhao, S.; Gan, X.Y.; Sun, A.; Xia, Y.; Liu, Y.J. Design of fluorescent hybrid materials based on POSS for sensing applications. Molecules 2022, 27, 3137. [Google Scholar] [CrossRef] [PubMed]
- Abumelha, H.M.; Alharbi, H.; Abualnaja, M.M.; Alsharief, H.H.; Ashour, G.R.; Saad, F.A.; El-Metwaly, N.M. Preparation of fluorescent ink using perylene-encapsulated silica nanoparticles toward authentication of documents. J. Photochem. Photobiol. A Chem. 2023, 441, 114706. [Google Scholar] [CrossRef]
- Qin, J.J.; Wang, H.; Xu, Y.; Shi, F.F.; Yang, S.J.; Huang, H.; Stewar, C.; Li, F.; Han, J.S.; Wu, W. A simple array integrating machine learning for identification of flavonoids in red wines. RSC Adv. 2023, 13, 8882–8889. [Google Scholar] [CrossRef]
- Kihal, N.; Nazemi, A.; Bourgault, S. Supramolecular nanostructures based on perylene diimide bioconjugates: From self-assembly to applications. Nanomaterials 2022, 12, 1223. [Google Scholar] [CrossRef]
- Rutschmann, M.; Feldmann, C. Perylene dye@SiO2 core–shell nanoparticles with intense fluorescence. J. Mater. Chem. C 2023, 11, 616–621. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, S.F.; Sun, Q.Q.; Xing, C.W.; Gao, W.; Lu, X.Q.; Li, X.Q.; Yang, G.W.; Yu, S.R.; Chen, Y.L. A perylenediimide modified SiO2@TiO2 yolk-shell light-responsive nanozyme: Improved peroxidase-like activity for H2O2 and sarcosine sensing. J. Hazard. Mater. 2022, 436, 129321. [Google Scholar] [CrossRef]
- Huth, K.; Glaeske, M.; Achazi, K.; Gordeev, G.; Kumar, S.; Arenal, R.; Sharma, S.K.; Adeli, M.; Setaro, A.; Reich, S.; et al. Fluorescent polymer—Single-walled carbon nanotube complexes with charged and noncharged dendronized perylene bisimides for bioimaging studies. Small 2018, 14, 1800796. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Keum, C.; Lee, S.G.; Lee, S.Y. Aggregation-driven fluorescence quenching of imidazole-functionalized perylene diimide for urea sensing. Analyst 2020, 145, 7312–7319. [Google Scholar] [CrossRef]
- Liao, C.L.; Zhang, M.; Tian, Q.Y.; Yang, X.M.; Shi, J.; Chen, S.; Che, Y.K.; Wang, C.Y.; Zang, L. Selective turn-on fluorescence detection of formaldehyde in the gas phase. Sens. Actuators B: Chem. 2023, 375, 132861. [Google Scholar] [CrossRef]
- Guan, W.J.; Zhou, W.J.; Lu, J.; Lu, C. Luminescent films for chemo- and biosensing. Chem. Soc. Rev. 2015, 44, 6981–7009. [Google Scholar] [CrossRef]





















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhou, M.; Zhu, L.; Yang, X.; Zang, L. Architectures and Mechanisms of Perylene Diimide-Based Optical Chemosensors for pH Probing. Chemosensors 2023, 11, 293. https://doi.org/10.3390/chemosensors11050293
Chen S, Zhou M, Zhu L, Yang X, Zang L. Architectures and Mechanisms of Perylene Diimide-Based Optical Chemosensors for pH Probing. Chemosensors. 2023; 11(5):293. https://doi.org/10.3390/chemosensors11050293
Chicago/Turabian StyleChen, Shuai, Meng Zhou, Ling Zhu, Xiaomei Yang, and Ling Zang. 2023. "Architectures and Mechanisms of Perylene Diimide-Based Optical Chemosensors for pH Probing" Chemosensors 11, no. 5: 293. https://doi.org/10.3390/chemosensors11050293