Changes in Foliar Functional Traits of S. pyrenaicus subsp. carpetanus under the Ongoing Climate Change: A Retrospective Survey
Abstract
:1. Introduction
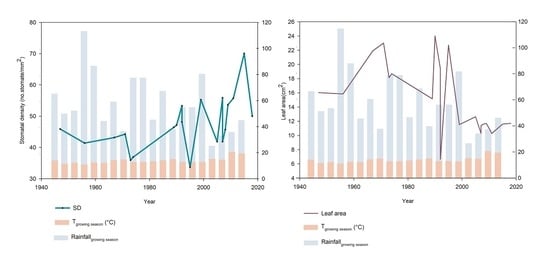
2. Results
Morphological and Micromorphological Parameters
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Plant Material
4.3. Stomatal and Other Leaf Traits
4.4. Climate Data and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McLaren, J.; Turkington, R. Ecosystem properties determined by plant functional group identity. J. Ecol. 2010, 98, 459–469. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 311, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species, 2nd ed.; Askew, A.P., Hodgson, J., Hunt, R., Eds.; Castlepoint Press: Dalbeattie, UK, 2007. [Google Scholar]
- Díaz, S.; Hodgson, J.G.; Thompon, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Marti, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef] [Green Version]
- McElwain, J.; Chaloner, W. Stomatal density and index of fossil plants track atmospheric carbon dioxide in the Paleozoic. Ann. Bot. 1995, 76, 389–395. [Google Scholar] [CrossRef]
- Suarez, A.; Tsutsui, N.D. The value of museum collections for research and society. BioScience 2004, 54, 66–74. [Google Scholar] [CrossRef]
- Royer, D. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 2001, 114, 1–28. [Google Scholar] [CrossRef]
- Lang, P.; Willems, F.M.; Scheepens, J.F.; Burbano, H.A.; Bossdorf, O. Using herbaria to study global environmental change. New Phytol. 2019, 221, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.; Dubbe, D.; Raschke, K. Gain of the feedback loop involving carbon dioxide and stomata. Theory and measurement. Plant Physiol. 1978, 62, 406–412. [Google Scholar] [CrossRef]
- Dow, G.; Bergmann, D. Patterning and processes: how stomatal development defines physiological potential. Curr. Opin. Plant Biol. 2014, 21, 67–74. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.; Beerling, D. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [Green Version]
- Ackerly, D.; Knight, C.A.; Weiss, S.B.; Barton, K.; Starmer, K.P. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Byars, S.; Papst, W.; Hoffmann, A. Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution 2007, 61, 2925–2941. [Google Scholar] [CrossRef]
- Franks, P.J.; Adams, M.A.; Amthor, J.S.; Barbour, M.M.; Berry, J.A.; Ellsworth, D.S.; Farquhar, G.D.; Ghannoum, O.; Lloyd, J.; McDowell, N.; et al. Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytol. 2013, 197, 1077–1094. [Google Scholar] [CrossRef]
- Körner, C.; Neumayer, M.; Menendezriedl, S.P.; Smeetsscheel, A. Functional Morphology of Mountain Plants. Flora 1989, 182, 353–383. [Google Scholar] [CrossRef]
- Bertolino, L.; Caine, R.; Gray, J. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Hayat, M.Q.; Ashraf, M.; Jabeen, S.; Shaheen, N.; Yasmin, G.; Khan, M.A. Taxonomic implications of foliar epidermal characteristics with special reference to stomatal variations in the genus Artemisia (Asteraceae). Int. J. Agric. Biol. 2010, 12, 221–226. [Google Scholar]
- Bano, A.; Ahmad, M.; Zafar, M.; Sultana, S.; Khan, M.A. Comparative foliar micromorphological studies of some species of Asteraceae from alpine zone of Deosai Plateau, Western Himalayas. J. Anim. Plant Sci. 2015, 25, 422–430. [Google Scholar]
- Fraser, L.; Greenall, A.; Carlyle, C.; Turkington, R.; Friedman, C.R. Adaptive phenotypic plasticity of Pseudoroegneria spicata: response of stomatal density, leaf area and biomass to changes in water supply and increased temperature. Ann. Bot. 2009, 103, 769–775. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Zhong, Y.; Shangguan, Z. Contrasting responses of leaf stomatal characteristics to climate change: a considerable challenge to predict carbon and water cycles. Glob. Change Biol. 2017, 23, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Matamala, R. Changes in N and S leaf content, stomatal density and specific leaf area of 14 plant species during the last three centuries of CO2 increase. J. Exp. Bot. 1990, 41, 1119–1124. [Google Scholar] [CrossRef]
- Kouwenberg, L.L.; Kürschner, K.W.; McElwain, J. Stomatal frequency change over altitudinal gradients: prospects for paleoaltimetry. Rev. Mineral. Geochem. 2007, 66, 215–241. [Google Scholar] [CrossRef]
- Guerin, G.R.; Wen, H.; Lowe, A.J. Leaf morphology shift linked to climate change. Biol. Lett. 2012, 8, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Labourdette, D.; Génova, M.; Schmitz, M.F.; Urrutia, R.; Pineda, F.D. Summer rainfall variability in European Mediterranean mountains from the sixteenth to the twentieth century reconstructed from tree rings. Int. J. Biometeorol. 2014, 58, 1627–1639. [Google Scholar] [CrossRef] [Green Version]
- Nogués-Bravo, D.; Araujo, M.B.; Lasanta, T.; Moreno, J.I.L. Climate Change in Mediterranean Mountains during the 21st Century. Ambio 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Fernández-González, F. Estudio Florístico y Fitosociológico del Valle del Paular Madrid. Ph.D. Thesis, Facultad de Biología, Universidad Complutense de Madrid, Madrid, Spain, 1988. [Google Scholar]
- Jiménez-Alfaro, B.; Gavilán, R.G.; Escudero, A.; Iriondo, J.M.; Fernández-González, F. Decline of dry grassland specialists in Mediterranean high-mountain communities influenced by recent climate warming. J. Veg. Sci. 2014, 25, 1394–1404. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Kazakis, G.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Liu, H.; Taylor, S.H.; Xu, Q.; Lin, Y.; Hou, H.; Wu, G.; Ye, Q. Life history is a key factor explaining functional trait diversity among subtropical grasses, and its influence differs between C3 and C4 species. J. Exp. Bot. 2019, 70, 1567–1580. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.F.; Amato, V.D.; Ricotta, C.; Frattaroli, A.R.; Puglielli, G. Leaf traits variation in Sesleria nitida growing at different altitudes in the Central Apennines. Photosynthetica 2014, 52, 386–396. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bresson, C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef]
- Michaletz, S.; Weiser, M.D.; McDowel, N.G.; Zhou, J.Z.; Kaspari, M.; Helliker, B.R.; Enquist, B.J. The energetic and carbon economic origins of leaf thermoregulation. Nat. Plants 2016, 2, 16129. [Google Scholar] [CrossRef] [PubMed]
- Roth-Nebelsick, A.; Konrad, W. Fossil leaf traits as archives for the past-and lessons for the future? Flora 2019, 254, 59–70. [Google Scholar] [CrossRef]
- Carlson, J.E.; Adams, C.A.; Holsinger, K.E. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Ann. Bot. 2016, 117, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Carlson, J.; Holsinger, K.; Prunier, R. Plant responses to climate in the Cape Floristic Region of South Africa: evidence for adaptive differentiation in the Proteaceae. Evolution 2011, 65, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Pauli, H.; Gottfried, M.; Grabher, G. Effect of climate change on the alpine and nival vegetation of the Alps. J. Mt. Ecol. 2003, 7, 9–12. [Google Scholar]
- Magaña Ugarte, R.; Escudero, A.; Gavilán, R.G. Couneracting summer-drought: Assessing the role of Pro and NSC accumulation in Mediterranean high-mountain plants. Frontiers 2020, in press. [Google Scholar]
- Deans, R.M.; Brodribb, T.J.; Busch, F.A.; Farquhar, G.D. Plant water-use strategy mediates stomatal effects on the light induction of photosynthesis. New Phytol. 2019, 222, 382–395. [Google Scholar] [CrossRef]
- Dittberner, H.; Korte, A.; Mettler-Altmann, T.; Weber, A.P.M.; Monroe, G.; de Meaux, J. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Mol. Ecol. 2018, 27, 4052–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellissier, L.; Brathen, K.A.; Pottier, J.; Randin, C.F.; Vittoz, P.; Dubui, A.; Yoccoz, N.G.; Alm, T.; Zimmermann, N.E.; Guisan, A. Species distribution models reveal apparent competitive and facilitative effects of a dominant species on the distribution of tundra plants. Ecography 2010, 33, 1004–1014. [Google Scholar] [CrossRef]
- Gutiérrez-Girón, A.; Gavilán, R.G. Plant functional strategies and environmental constraints in Mediterranean high mountain grasslands in central Spain. Plant Ecol. Div. 2013, 6, 435–446. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Plantaginaceae to Compositae (and Rubiaceae); Cambridge University Press: Cambridge, UK, 1976; Volume 4, p. 534. [Google Scholar]
- Weyers, J.; Johansen, L. Accurate estimation of stomatal aperture from silicone rubber impressions. New Phytol. 1985, 101, 109–115. [Google Scholar] [CrossRef]
- Fanourakis, D.; Heuvelink, E.; Carvalho, S. A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. J. Plant Physiol. 2013, 170, 890–898. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize Results of Multivariate Data Analyses, R package version 1.0.5; R Foundation for Statistical Computing: Vienna, Austria; p. 2017.
- Wood, S. Fast stable restricted maximum likelihood estimation of semi parametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña Ugarte, R.; Escudero, A.; Sánchez Mata, D.; Gavilán, R.G. Changes in Foliar Functional Traits of S. pyrenaicus subsp. carpetanus under the Ongoing Climate Change: A Retrospective Survey. Plants 2020, 9, 395. https://doi.org/10.3390/plants9030395
Magaña Ugarte R, Escudero A, Sánchez Mata D, Gavilán RG. Changes in Foliar Functional Traits of S. pyrenaicus subsp. carpetanus under the Ongoing Climate Change: A Retrospective Survey. Plants. 2020; 9(3):395. https://doi.org/10.3390/plants9030395
Chicago/Turabian StyleMagaña Ugarte, Rosina, Adrián Escudero, Daniel Sánchez Mata, and Rosario G. Gavilán. 2020. "Changes in Foliar Functional Traits of S. pyrenaicus subsp. carpetanus under the Ongoing Climate Change: A Retrospective Survey" Plants 9, no. 3: 395. https://doi.org/10.3390/plants9030395