Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn
Abstract
:1. Introduction
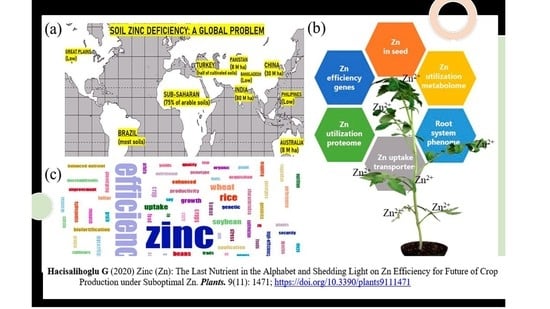
2. Soil Zn Deficiency
3. Evidence of Natural Genetic Variation for Plant Zn Efficiency: A Large Untapped Resource for Overcoming Low-Zn Stress
4. Zn Efficiency Strategies in Crop Plants
4.1. Plant Zn efficiency Mechanism Candidate 1—Zn Uptake Systems and Transporters of Zn
4.2. Plant Zn Efficiency Mechanism Candidate 2—Shoot Internal Zn Utilization
4.3. Other Mechanisms
5. Conclusions, Future Challenges, and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Statistical Databases. Available online: http://apps.fao.org/ (accessed on 20 September 2020).
- Welch, R.M.; Graham, R.D. Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. J. Trace Elem. Med. Biol. 2005, 18, 299–307. [Google Scholar] [CrossRef]
- Wessels, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national foods supplies and prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995; p. 889. [Google Scholar]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Hacisalihoglu, G.; Kochian, L.V. How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytol. 2004, 159, 341–350. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Blair, M. Current advances in zinc in soils and plants: Implications for zinc efficiency and biofortification studies. Achiev. Sustain. Crop Nutr. 2020, 76, 337–353. [Google Scholar]
- Hacisalihoglu, G.; Hart, J.J.; Kochian, L.V. High and low affinity Zn transport systems and their possible role in Zn efficiency in bread wheat. Plant Physiol. 2001, 125, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Torun, B.; Erenoglu, B.; Oztürk, L.; Marschner, H.; Kalayci, M.; Ekiz, H.; Yilmaz, A. Morphological and physiological differences in the response of cereals to zinc deficiency. Euphytica 1998, 100, 349–357. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Ozturk, L.; Cakmak, I.; Welch, R.M.; Kochian, L.V. Genotypic variation in common bean in response to Zn deficiency in calcareous soil. Plant Soil 2004, 259, 71–83. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu. Rev. Plant Biol. 1999, 50, 133–161. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification. Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Sommer, A.L.; Lipman, C.B. Evidence on indispensable nature of zinc and boron for higher green plants. Plant Physiol. 1926, 1, 231–249. [Google Scholar] [CrossRef]
- Kochian, L.V. Zinc absorption from hydroponic solutions by plant roots. In Zinc in Soils and Plants; Kluwer Academic Publishers: Berlin, Germany, 1993; pp. 45–57. [Google Scholar]
- Neilsen, G.H.; Neilsen, D. Tree Fruit Zinc Nutrition; GoodFruit Grower: Yakima, WA, USA, 1994; pp. 85–93. [Google Scholar]
- Koo, R.C.J. Fertilization and Irrigation Effects on Fruit Quality. Factors Affect. Fruit Qual. Citrus Short Course Proc. 1988, 97, 35–42. [Google Scholar]
- Khan, H.R.; McDonald, G.K.; Rengel, Z. Chickpea genotypes differ in their sensitivity to Zn deficiency. Plant Soil 1998, 198, 11–18. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Rehman, A.; Hussain, M.; Siddique, K.H.M. Zinc nutrition in chickpea (Cicer arietinum): A review. Crop Pasture Sci. 2020, 71, 199–218. [Google Scholar] [CrossRef]
- Fageria, N.K. Screening method of lowland rice genotypes for zinc uptake efficiency. Sci. Agric. 2001, 58, 623–626. [Google Scholar] [CrossRef]
- Naik, S.M.; Raman, A.K.; Nagamallika, M.; Venkateshwarlu, C.; Singh, S.P.; Kumar, S.; Singh, S.K.; Tomizuddin, A.; Das, S.P.; Prasad, K.; et al. Genotype × environment interactions for grain iron and zinc content in rice. J. Sci. Food Agric. 2020, 100, 4150–4164. [Google Scholar] [CrossRef]
- Shukla, U.C.; Raj, H. Zinc response in corn as influenced by genetic variability. Agron. J. 1976, 68, 20–22. [Google Scholar] [CrossRef]
- Shukla, U.C.; Arora, S.K.; Singh, Z.; Prasad, K.G.; Safaya, N.M. Differential susceptibility of some sorghum genotypes to zinc deficiency in soil. Plant Soil 1973, 39, 423–427. [Google Scholar] [CrossRef]
- Graham, R.D.; Rengel, Z. Genotypic variation in zinc uptake and utilization by plants. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 107–118. [Google Scholar]
- Genc, Y.; McDonald, G.K.; Rengel, Z.; Graham, R.D. Genotypic variation in the response of barley to Zn deficiency. In Plant Nutrition-Molecular Biology and Genetics; Gissel-Nielsen, G., Ed.; Kluwer Publishers: Dordrecht, The Netherlands, 1999; pp. 205–221. [Google Scholar]
- Behera, S.K.; Shukla, A.K.; Tiwari, P.K.; Tripathi, A.; Singh, P.; Trivedi, V.; Patra, A.K.; Das, S. Classification of Pigeonpea (Cajanus cajan (L.) Millsp.) Genotypes for Zinc Efficiency. Plants 2020, 9, 952. [Google Scholar] [CrossRef]
- Genc, Y.; Humphries, J.M.; Lyons, G.H.; Graham, R.D. Exploiting genotypic variation in plant nutrient accumulation to alleviate micronutrient deficiency in populations. J. Trace Elem. Med. Biol. 2005, 18, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zou, C.; Zhang, F.; vander Zee, E.; Hoffland, E. Tolerance to zinc deficiency in rice correlates with zinc uptake and translocation. Plant Soil 2005, 278, 253–261. [Google Scholar] [CrossRef]
- Cakmak, O.; Ozturk, L.; Karanlik, S.; Ozkan, H.; Kaya, Z.; Cakmak, I. Tolerance of 65 durum wheat genotypes to zinc deficiency in a calcareous soil. J. Plant Nutr. 2001, 24, 1831–1847. [Google Scholar] [CrossRef]
- Lu, X.C.; Cui, J.; Tian, X.H.; Ogunniyi, J.E.; Gale, W.J.; Zhao, A.Q. Effects of zinc fertilization on zinc dynamics in potentially zinc-deficient calcareous soil. Agron. J. 2012, 104, 963–969. [Google Scholar] [CrossRef]
- Siqueira, O.J.F. Response of Soybeans and Wheat to Limestone Application on Acid Soils in RioGrande do Sul, Brazil; Digital Repository Iowa State University: Ames, IA, USA, 1977; p. 224. [Google Scholar]
- Genc, Y.; McDonald, G.K.; Graham, R.D. Contribution of different mechanisms to zinc efficiency in bread wheat during early vegetative stage. Plant Soil 2006, 281, 353–367. [Google Scholar] [CrossRef]
- Blair, M.; Astudillo, C.; Grusak, M.; Graham, R.; Beebe, S. Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- QuickStats. USDA-NASS, State Agriculture Overview: Arkansas. 2019. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=ARKANSAS (accessed on 20 September 2020).
- Norman, R.J.; Wilson, C.E.; Slaton, N.A. Soil fertilization and mineral nutrition in US mechanized rice culture. In Rice: Origin, History, Technology, and Production; Wiley: Hoboken, NJ, USA, 2003; pp. 331–412. [Google Scholar]
- Maze, P. Influences respective des elements de la solution minérale sur le development du mais. Ann. Inst. Pasteur 1914, 28, 21–68. [Google Scholar]
- Furlani, A.M.C.; Furlani, P.R.; Meda, A.R.; Durate, A.P. Efficiency of maize cultivars for zinc uptake and use. Sci. Agric. 2005, 62, 264–273. [Google Scholar] [CrossRef]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Welch, R.M. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1993, 14, 49–87. [Google Scholar] [CrossRef]
- Rengel, Z. Physiological mechanisms underlying differential nutrient efficiency of crop genotypes. In Mineral Nutrition of Crops; Food Products Press: Binghamton, NY, USA, 1999; pp. 231–261. [Google Scholar]
- Barber, S. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Rengel, Z.; Graham, R.D. Uptake of zinc from chelate-buffered nutrient solutions by wheat genotypes differing in zinc efficiency. J. Exp. Bot. 1996, 47, 217–226. [Google Scholar] [CrossRef]
- Milner, M.J.; Craft, E.; Yamaji, N.; Ma, J.F.; Kochian, L.V. Characterization of the high affinity Zn transporter from Noccaea caerulescens, NcZNT1, and dissection of its promoter for its role in Zn uptake and hyperaccumulation. New Phytol. 2012, 195, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tiong, J.; McDonald, G.K.; Genc, Y.; Pedas, P.; Hayes, J.E.; Toubia, J.; Langridge, P.; Huang, C.Y. HvZIP7 mediates zinc accumulation in barley (Hordeum vulgare) at moderately high zinc supply. New Phytol. 2014, 201, 131–143. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta 2000, 1465, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Assuncao, A.G.L.; Herrero, E.; Lin, Y.F.; Huettel, B.; Talukdar, S.; Smaczniak, C.; Immink, G.H.; van Eldik, M.; Fiers, M.; Schat, H.; et al. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 10296–10301. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Kawachi, M.; Sato, Y.; Mori, H.; Kutsuna, N.; Hasezawa, S.; Maeshima, M. A high molecular mass zinc transporter MTP12 forms a functional heteromeric complex with MTP5 in the Golgi in Arabidopsis thaliana. FEBS J. 2015, 282, 1965–1979. [Google Scholar] [CrossRef]
- Kramer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef]
- Huang, S.; Sasaki, A.; Yamaji, N.; Okada, H.; Mitani-Ueno, N.; Ma, J.F. The ZIP Transporter Family Member OsZIP9 Contributes to Root Zinc Uptake in Rice under Zinc-Limited Conditions. Plant Physiol. 2020, 183, 1224–1234. [Google Scholar] [CrossRef]
- Zhang, X.K.; Zhang, F.S.; Mao, D.R. Effect of iron plaque outside roots on nutrient uptake by rice (Oryza sativa L.): Zinc uptake by Fe-deficient rice. Plant Soil 1998, 202, 33–39. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Hart, J.J.; Vallejos, C.E.; Kochian, L.V. The Role of shoot-localized processes in the mechanism of Zn efficiency in common bean. Planta 2004, 218, 704–711. [Google Scholar] [CrossRef]
- Frei, M.; Wang, Y.; Ismail, A.M.; Wissuwa, M. Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Funct. Plant Biol. 2010, 37, 74–84. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Hart, J.J.; Cakmak, I.; Wang, Y.; Kochian, L.V. Zinc Efficiency is correlated with enhanced expression and activities of of Cu/Zn-SOD and carbonic anhydrase in wheat. Plant Physiol. 2003, 131, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Ozturk, L.; Eker, S.; Torun, B.; Kalfa, H.I.; Yilmaz, A. Concentration of Zn and activity of copper/zinc superoxide dismutase in leaves of rye and wheat cultivars differing in sensitivity to zinc deficiency. J. Plant Physiol. 1997, 151, 91–95. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, A.K.; Behera, S.K.; Tiwari, P.K. Zinc applicationenhances super oxide dismutase and carbonic anhydrase activities in zinc efficient and inefficient wheat genotypes. J. Soil. Sci. Plant Nutr. 2019, 19, 477–487. [Google Scholar] [CrossRef]
- Blair, M.W.; Izquierdo, P. Use of the advanced backcross-QTL method to transfer seed mineral accumulation nutrition traits from wild to Andean cultivated common beans. Theor. Appl. Genet. 2012, 125, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, H.; Tong, Y.; Jing, R.; Zhang, F.; Zou, C. Identification of quantitative trait locus of zinc and phosphorus density in wheat (Triticum aestivum L.) grain. Plant Soil 2008, 306, 95–104. [Google Scholar] [CrossRef]
- Stangoulis, J.C.R.; Huynh, B.L.; Welch, R.M.; Choi, E.Y.; Graham, R.D. Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica 2007, 154, 289–294. [Google Scholar] [CrossRef]
- Simic, D.; Mladenovic Drinic, S.; Zdunic, Z.; Jambrovic, A.; Ledencan, T.; Brkic, J.; Brkic, A.; Brkic, I. Quantitative trait Loci for biofortification traits in maize grain. J. Hered. 2012, 103, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Gelin, J.R.; Forster, S.; Grafton, K.F.; McClean, P.; Rojas-Cifuentes, G.A. Analysis of seed-zinc and other nutrients in a recombinant inbred population of navy bean (Phaseolus vulgaris L.). Crop Sci. 2007, 47, 1361–1366. [Google Scholar] [CrossRef]
- Huang, F.; Wei, X.; He, J.; Sheng, Z.; Shao, G.; Wang, J.; Tang, S.; Xia, S.; Xiao, Y.; Hu, P. Mapping of quantitative trait loci associated with concentrations of five trace metal elements in rice (Oryza sativa). Int. J. Biol. 2018, 20, 554–560. [Google Scholar]
- Velu, G.; Tutus, Y.; Gomez-Becerra, H.F.; Hao, Y.; Demir, L.; Kara, R. QTL mapping for grain zinc and iron concentrations and zinc efficiency in a tetraploid and hexaploid wheat mapping populations. Plant Soil 2017, 411, 81–99. [Google Scholar] [CrossRef]
- Jeong, O.Y.; Bombay, M.; Ancheta, M.B.; Lee, J.H. QTL for the iron and zinc contents of the milled grains of a doubled-haploid rice (Oryza sativa L.) population grown over two seasons. J. Crop Sci. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacisalihoglu, G. Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn. Plants 2020, 9, 1471. https://doi.org/10.3390/plants9111471
Hacisalihoglu G. Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn. Plants. 2020; 9(11):1471. https://doi.org/10.3390/plants9111471
Chicago/Turabian StyleHacisalihoglu, Gokhan. 2020. "Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn" Plants 9, no. 11: 1471. https://doi.org/10.3390/plants9111471