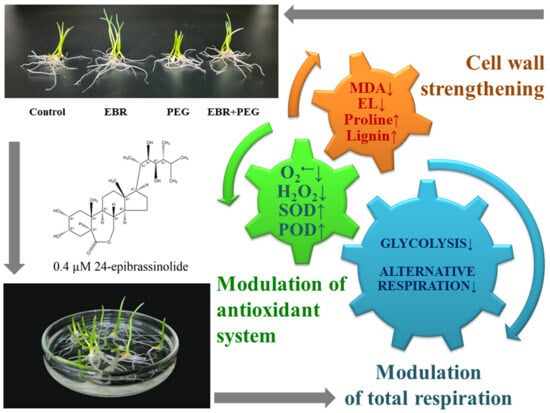
24-Epibrassinolide Reduces Drought-Induced Oxidative Stress by Modulating the Antioxidant System and Respiration in Wheat Seedlings
Abstract
:1. Introduction
2. Results
2.1. Effect of EBR Application on the Growth of Wheat Seedlings under Drought Stress
2.2. Effect of EBR on O2•− and H2O2 Production and the Activity of Antioxidant Enzymes under Normal and Drought Conditions
2.3. Effect of EBR on Proline under Normal and Drought Conditions
2.4. Effect of EBR on the Respiration Parameters of Wheat Seedlings under Normal and Drought Conditions
2.5. Lignin Deposition in EBR-Pretreated and EBR-Untreated Wheat Seedlings under Normal and Drought Conditions
2.6. Influence of Drought Stress on Membrane Lipid Peroxidation and Electrolyte Leakage of Wheat Seedlings Untreated and Pretreated with EBR
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Growth Rate Analysis
4.3. Determination of Superoxide Radical and Hydrogen Peroxide Production
4.4. Determination of Antioxidant Enzymes Activities
4.5. Evaluation of Lignin Deposition
4.6. Estimation of Malondialdehyde and Electrolyte Leakage
4.7. Proline Determination
4.8. Determination of Respiration Parameters
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Díaz, K.; Espinoza, L.; Carvajal, R.; Silva-Moreno, E.; Olea, A.F.; Rubio, J. Exogenous Application of Brassinosteroid 24-Norcholane 22(S)-23-Dihydroxy Type Analogs to Enhance Water Deficit Stress Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 1158. [Google Scholar] [CrossRef]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Ostrowska, A. Drought-stress induced physiological and molecular changes in plants 2.0. Int. J. Mol. Sci. 2023, 24, 1773. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- González, E.M. Drought stress tolerance in plants. Int. J. Mol. Sci. 2023, 24, 6562. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Khripach, V.; Zhabinskii, V.; de Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Sasse, J.M. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Tang, J.; Han, Z.; Chai, J. Q&A: What are brassinosteroids and how do they act in plants? BMC Biol. 2016, 14, 113. [Google Scholar] [CrossRef]
- Lanctot, A. Branching out underground: Brassinosteroid signaling promotes lateral root development in rice. Plant Physiol. 2022, 189, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Wang, Z.Y. Brassinosteroid action in flowering plants: A Darwinian perspective. J. Exp. Bot. 2012, 63, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Sadura, I.; Janeczko, A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant 2018, 62, 601–616. [Google Scholar] [CrossRef]
- Müssig, C.; Altmann, T. Genomic brassinosteroid effects. J. Plant Growth Regul. 2003, 22, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Sieprawska, A.; Kościelniak, J.; Oklestkova, J.; Jurczyk, B.; Telk, A.; Biesaga-Kościelniak, J.; Janeczko, A. The role of chloroplasts in the oxidative stress that is induced by zearalenone in wheat plants—The functions of 24-epibrassinolide and selenium in the protective mechanisms. Plant Physiol. Biochem. 2019, 137, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Russinova, E. Brassinosteroid signalling. Curr. Biol. 2020, 30, R294–R298. [Google Scholar] [CrossRef]
- Jager, C.E.; Symons, G.M.; Ross, J.J.; Reid, J.B. Do brassinosteroids mediate the water stress response? Physiol. Plant. 2008, 133, 417–425. [Google Scholar] [CrossRef]
- Gruszka, D. Exploring the Brassinosteroid Signaling in Monocots Reveals Novel Components of the Pathway and Implications for Plant Breeding. Int. J. Mol. Sci. 2020, 21, 354. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Ahmad Lone, W.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Xie, R.; Zhou, C.; Wu, X.; Li, D. Roles of Brossinosteroids Signaling in Biotic and Abiotic Stresses. J. Agric. Food Chem. 2023, 71, 7947–7960. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Dehghan, M.; Balouchi, H.; Yadavi, A.; Zare, E. Improve wheat (Triticum aestivum) performance by brassinolide application under different irrigation regimes. S. Afr. J. Bot. 2020, 130, 259–267. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Ibrahim, M.A.; Ditta, A.; Iqbal, R.; Aslam, M.U.; Muhammad, F.; Ali, S.; Çiğ, F.; Ali, B.; Muhammad Ikram, R.; et al. Exploring the recuperative potential of brassinosteroids and nano-biochar on growth, physiology, and yield of wheat under drought stress. Sci. Rep. 2023, 13, 15015. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Gao, Y.; Guo, J.; Yu, T.-F.; Zheng, W.-J.; Liu, Y.-W.; Chen, J.; Xu, Z.-S.; Ma, Y.-Z. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-Induced Reactive Oxygen Species Accumulation by Altering the Enzymatic Antioxidant System and Associated Cytotoxic, Genotoxic, Ultrastructural, and Photosynthetic Changes in Plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Advances in the understanding of reactive oxygen species-dependent regulation on seed dormancy, germination, and deterioration in crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Luo, Z.; Zeng, F.; Jiang, L.; Tang, K. Effect of brassinolide on energy status and proline metabolism in postharvest bamboo shoot during chilling stress. Postharvest Biol. Technol. 2016, 111, 240–246. [Google Scholar] [CrossRef]
- Yu, J.Q.; Huang, L.F.; Hu, W.H.; Zhou, Y.H.; Mao, W.H.; Ye, S.F.; Nogueas, S. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J. Exp. Bot. 2004, 399, 1135–1143. [Google Scholar] [CrossRef]
- Xia, X.-J.; Huang, L.-F.; Zhou, Y.-H.; Mao, W.-H.; Shi, K.; Wu, J.-X.; Asami, T.; Chen, Z.; Yu, J.-Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Derevyanchuk, M.V.; Grabelnyh, O.I.; Litvinovskaya, R.P.; Voinikov, V.K.; Sauchuk, A.L.; Khripach, V.A.; Kravets, V.S. Influence of brassinosteroids on plant cell alternative respiration pathway and antioxidant systems activity under abiotic stress conditions. Biopolym. Cell 2014, 30, 436–442. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.G.; Tan, W.R.; Zhou, X.; Luo, S.S.; Han, X.Y.; Zhang, D.W.; Lin, H.H. Nitric oxide is involved in brassinosteroid-induced alternative respiratory pathway in Nicotiana benthamiana seedlings’ response to salt stress. Physiol. Plant 2016, 156, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef]
- Rashid, F.A.A.; Crisp, P.A.; Zhang, Y.; Berkowitz, O.; Pogson, B.J.; Day, D.A.; Masle, J.; Dewar, R.C.; Whelan, J.; Atkin, O.K.; et al. Molecular and physiological responses during thermal acclimation of leaf photosynthesis and respiration in rice. Plant Cell Environ. 2020, 43, 594–610. [Google Scholar] [CrossRef]
- Mujawamariya, M.; Wittemann, M.; Manishimwe, A.; Ntirugulirwa, B.; Zibera, E.; Nsabimana, D.; Wallin, G.; Uddling, J.; Dusenge, M.E. Complete or overcompensatory thermal acclimation of leaf dark respiration in African tropical trees. New Phytol. 2021, 229, 2548–2561. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Fan, Y.; Posch, B.C.; Garcia, A.; Coast, O.; Atkin, O.K. Responses of leaf respiration to heatwaves. Plant Cell Environ. 2021, 44, 2090–2101. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C. Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Dahal, K.; Alber, N.A.; Chadee, A. Photosynthesis, respiration and growth: A carbon and energy balancing act for alternative oxidase. Mitochondrion 2020, 52, 197–211. [Google Scholar] [CrossRef]
- Manbir; Singh, P.; Kumar, A.; Kapuganti, J.G. Alternative oxidase plays a role in minimizing ROS and RNS produced under salinity stress in Arabidopsis thaliana. Physiol. Plant 2022, 174, e13649. [Google Scholar] [CrossRef]
- He, Q.; Wang, X.; He, L.; Yang, L.; Wang, S.; Bi, Y. Alternative respiration pathway is involved in the response of highland barley to salt stress. Plant Cell Rep. 2019, 38, 295–309. [Google Scholar] [CrossRef]
- Hua, D.; Ma, M.; Ge, G.; Suleman, M.; Li, H. The role of cyanide-resistant respiration in Solanum tuberosum L. against high light stress. Plant Biol. 2020, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Watling, J.R.; Robinson, S.A.; Seymour, R.S. Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus. Plant Physiol. 2006, 140, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Krab, K.; Wagner, M.J.; Moore, A.L. Regulation of thermogenesis in flowering Araceae: The role of the alternative oxidase. Biochim. Biophys. Acta 2008, 1777, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yuan, S.; Zhang, D.-W.; Lv, X.; Lin, H.-H. The role of alternative oxidase in tomato fruit ripening and its regulatory interaction with ethylene. J. Exp. Bot. 2012, 63, 5705–5716. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Wang, Y.; Xing, D. Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria-dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis. J. Exp. Bot. 2014, 65, 4465–4478. [Google Scholar] [CrossRef]
- Deng, X.G.; Zhu, T.; Zhang, D.W.; Lin, H.H. The alternative respiratory pathway is involved in brassinosteroid-induced environmental stress tolerance in Nicotiana benthamiana. J. Exp. Bot. 2015, 66, 6219–6232. [Google Scholar] [CrossRef]
- Avalbaev, A.; Yuldashev, R.; Fedorova, K.; Petrova, N.; Fedina, E.; Gilmanova, R.; Karimova, F.; Shakirova, F. 24-epibrassinolide-induced growth promotion of wheat seedlings is associated with changes in the proteome and tyrosine phosphoproteome. Plant Biol. 2021, 23, 456–463. [Google Scholar] [CrossRef]
- Avalbaev, A.M.; Yuldashev, R.A.; Fatkhutdinova, R.A.; Urusov, F.A.; Safutdinova, Y.V.; Shakirova, F.M. The influence of 24-epibrassidinolide on the hormonal status of wheat plants under sodium chloride. Appl. Biochem. Microbiol. 2010, 46, 99–102. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. Int. 2021, 28, 44768–44779. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Khripach, V.A.; Kuznetsov, V.V.; Efimova, M.V. Comparison of protective reactions of rape seeds to chloride salination at exposure to epibrassinolide before or during salt stress. Dokl. Biochem. Biophys. 2022, 502, 25–29. [Google Scholar] [CrossRef]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Koul, K.K.; Srivastava, N. Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol. Mol. Biol. Plants 2019, 25, 683–696. [Google Scholar] [CrossRef]
- Singh, S.K.; Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Hydrogen sulphide ameliorates hexavalent chromium toxicity in two cereal crops: Role of antioxidant enzymes and proline metabolism. Plant Biol. 2022, 24, 636–641. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 17, 17–23. [Google Scholar] [CrossRef]
- Merwad, A.R.M.; Desoky, E.S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Guan, C.; Cui, X.; Liu, H.Y.; Li, X.; Li, M.Q.; Zhang, Y.W. Proline biosynthesis enzyme genes confer salt tolerance to switchgrass (Panicum virgatum L.) in cooperation with polyamines metabolism. Front. Plant Sci. 2020, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.; Bor, M.; Demiral, T.; Türkan, İ. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul. 2004, 42, 203–211. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; Viana, J.O.F.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Ménard, D.; Blaschek, L.; Kriechbaum, K.; Lee, C.C.; Serk, H.; Zhu, C.; Lyubartsev, A.; Nuoendagula; Bacsik, Z.; Bergström, L.; et al. Plant biomechanics and resilience to environmental changes are controlled by specific lignin chemistries in each vascular cell type and morphotype. Plant Cell 2022, 34, 4877–4896. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J.S. Modulation of lignin biosynthesis for drought tolerance in plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef]
- Rakhmankulova, Z.F.; Fedyaev, V.V.; Podashevka, O.A.; Usmanov, I.Y. Alternative respiration pathways and secondary metabolism in plants with different adaptive strategies under mineral deficiency. Russ. J. Plant Physiol. 2003, 50, 206–212. [Google Scholar] [CrossRef]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q.; Zhang, D.W.; Lin, H.H. Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Interactions of brassinosteroids with major phytohormones: Antagonistic effects. J. Plant Growth Regul. 2018, 37, 1025–1032. [Google Scholar] [CrossRef]
- Saha, B.; Borovskii, G.; Panda, S.K. Alternative oxidase and plant stress tolerance. Plant Signal. Behav. 2016, 11, e1256530. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Sugie, A.; Kobayashi, F.; Takumi, S. Mitochondrial alternative pathway is associated with development of freezing tolerance in common wheat. J. Plant Physiol. 2008, 165, 462–467. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahoo, L.; Katsuhara, M.; Matsumoto, H. Overexpression of alternative oxidase gene confers aluminum tolerance by altering the respiratory capacity and the response to oxidative stress in tobacco cells. Mol. Biotechnol. 2013, 54, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Duan, J.; Li, H.; Liang, H.; Li, X.; Han, N. Alternative respiratory pathway under drought is partially mediated by hydrogen peroxide and contributes to antioxidant protection in wheat leaves. Plant Prod. Sci. 2008, 11, 59–66. [Google Scholar] [CrossRef]
- Grabelnych, O.I.; Borovik, O.A.; Tauson, E.L.; Pobezhimova, T.P.; Katyshev, A.I.; Pavlovskaya, N.S.; Koroleva, N.A.; Lyubushkina, I.V.; Bashmakov, V.Y.; Popov, V.N.; et al. Mitochondrial energy-dissipating systems (alternative oxidase, uncoupling proteins, and external NADH dehydrogenase) are involved in development of frost-resistance of winter wheat seedlings. Biochemistry 2014, 79, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Garmash, E.V.; Golovko, T.K. Effect of cadmium on growth and respiration of barley plants grown under two temperature regimes. Russ. J. Plant Physiol. 2009, 56, 343–347. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.W.; Tu, S.H.; Feng, W.Q.; Xu, F.; Zhu, F.; Zhang, D.W.; Du, J.B.; Yuan, S.; Lin, H.H. Comparative study of four rice cultivars with different levels of cadmium tolerance. Biologia 2013, 68, 74–81. [Google Scholar] [CrossRef]
- Keunen, E.; Schellingen, K.; Van Der Straeten, D.; Remans, T.; Colpaert, J.; Vangronsveld, J.; Cuypers, A. ALTERNATIVE OXIDASE1a modulates the oxidative challenge during moderate Cd exposure in Arabidopsis thaliana leaves. J. Exp. Bot. 2015, 66, 2967–2977. [Google Scholar] [CrossRef]
- Gunn, S.; Farrar, J.F. Effects of a 40 C increase in temperature on partitioning of leaf area and dry mass, root respiration and carbohydrates. Funct. Ecol. 1999, 13, 12–20. [Google Scholar] [CrossRef]
- Rachmilevitch, S.; Lambers, H.; Huang, B. Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. J. Exp. Bot. 2006, 57, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Bezrukova, M.V.; Aval’baev, A.M.; Gimalov, F.R. Stimulation of Wheat Germ Agglutinin Gene Expression in Root Seedlings by 24-Epibrassinolide. Russ. J. Plant Physiol. 2002, 49, 225–228. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Minibayeva, F.V.; Gordon, L.K.; Kolesnikov, O.P.; Chasov, A.V. Role of extracellular peroxidase in the superoxide production by wheat root cells. Protoplasma 2001, 217, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.V.; Burkhanova, G.F.; Nuzhnaya, T.V.; Maksimov, I.V. Roles of ethylene and cytokinins in development of defense responses in Triticum aestivum plants infected with Septoria nodorum. Russ. J. Plant Physiol. 2016, 63, 609–619. [Google Scholar] [CrossRef]
- Beyer, Y.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Yusupova, Z.R.; Akhmetova, I.E.; Khairullin, R.M.; Maksimov, I.V. The effect of chitooligosaccharides on hydrogen peroxidase production and anionic peroxidase activity in wheat coleoptiles. Russ. J. Plant Physiol. 2005, 52, 209–213. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tao, S.; Khanizadeh, S.; Zhang, H.; Zhang, S. Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Sci. 2009, 176, 413–419. [Google Scholar] [CrossRef]
- Bezrukova, M.; Kildibekova, A.; Shakirova, F. WGA reduces the level of oxidative stress in wheat seedlings under salinity. Plant Growth Regul. 2008, 54, 195–201. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldran, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Rakhmankulova, Z.F.; Fedyaev, V.V.; Rakhmatulina, S.R.; Ivanov, C.P.; Gilvanova, I.R.; Usmanov, I.Y. The effect of wheat seed presowing treatment with salicylic acid on its endogenous content, activities of respiratory pathways, and plant antioxidant status. Russ. J. Plant Physiol. 2010, 57, 778–783. [Google Scholar] [CrossRef]





| Variant | 5th Day |
|---|---|
| Control | – |
| EBR | + |
| 12% PEG | + |
| EBR + 12% PEG | ++ |
| Variant | MDA Content, mol g−1 Fresh Weight | Electrolyte Leakage, µS/g Fresh Weight |
|---|---|---|
| Control | 40.8 ± 2.0 c | 20.1 ± 1.3 c |
| EBR | 42.2 ± 1.9 c | 21.9 ± 1.4 c |
| 12% PEG | 95.5 ± 4.2 a | 47.8 ± 2.3 a |
| EBR + 12% PEG | 65.1 ± 3.1 b | 32.3 ± 1.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avalbaev, A.; Fedyaev, V.; Lubyanova, A.; Yuldashev, R.; Allagulova, C. 24-Epibrassinolide Reduces Drought-Induced Oxidative Stress by Modulating the Antioxidant System and Respiration in Wheat Seedlings. Plants 2024, 13, 148. https://doi.org/10.3390/plants13020148
Avalbaev A, Fedyaev V, Lubyanova A, Yuldashev R, Allagulova C. 24-Epibrassinolide Reduces Drought-Induced Oxidative Stress by Modulating the Antioxidant System and Respiration in Wheat Seedlings. Plants. 2024; 13(2):148. https://doi.org/10.3390/plants13020148
Chicago/Turabian StyleAvalbaev, Azamat, Vadim Fedyaev, Alsu Lubyanova, Ruslan Yuldashev, and Chulpan Allagulova. 2024. "24-Epibrassinolide Reduces Drought-Induced Oxidative Stress by Modulating the Antioxidant System and Respiration in Wheat Seedlings" Plants 13, no. 2: 148. https://doi.org/10.3390/plants13020148