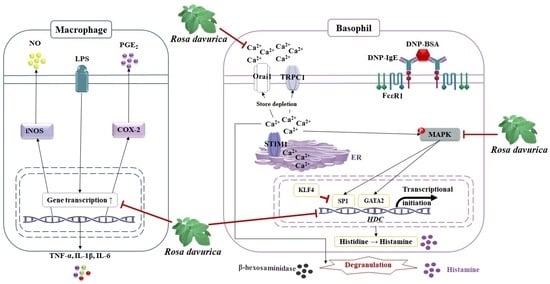
Rosa davurica Inhibited Allergic Mediators by Regulating Calcium and Histamine Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Chemical Profiling of RLE
2.2. Effects of RLE on Cell Viability
2.3. Effects of RLE Extract on NO Production in RAW 264.7 Cells
2.4. Effects of RLE on Cell Degranulation and Histamine Release from RBL-2H3 Cells
2.5. Effects of RLE on Calcium Influx in RBL-2H3 Cells
2.6. Effects of RLE Extract on the mRNA Expression of Inflammatory Mediators in RAW 264.7 Cells
2.7. Effects of RLE on the mRNA and Protein Expression of HDC and HDC Transcription Factors
2.8. Effects of RLE on the Protein Expression of MAPK Pathways
2.9. Effects of RLE on the Protein Expression of Calcium Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Sample Preparations
4.3. Chemical Profiling by High-Performance Liquid Chromatography
4.4. Cell Culture
4.5. Sample Treatment
4.6. Measurement of Cell Viability
4.7. Measurement of NO Production
4.8. β-Hexosaminidase Release Assay
4.9. Histamine Release Assay
4.10. Measurement of Intracellular Calcium Levels
4.11. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sicherer, S.H.; Leung, D.Y. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insect stings. J. Allergy Clin. Immunol. 2004, 114, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef]
- Kanagaratham, C.; El Ansari, Y.S.; Lewis, O.L.; Oettgen, H.C. Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front. Immunol. 2020, 11, 603050. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, E.; Ryu, S.H.; Suh, P.G. The mechanism of phospholipase C-gamma1 regulation. Exp. Mol. Med. 2000, 32, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampieri, A.; Santoyo, K.; Asanov, A.; Vaca, L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep. 2018, 8, 13252. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular Regulation of Histamine Synthesis. Front. Immunol. 2018, 9, 1392. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, T.; Takai, J.; Moriguchi, T.; Takai, J. Histamine and histidine decarboxylase: Immunomodulatory functions and regulatory mechanisms. Genes Cells 2020, 25, 443–449. [Google Scholar] [CrossRef]
- Ai, W.; Liu, Y.; Langlois, M.; Wang, T.C. Kruppel-like factor 4 (KLF4) represses histidine decarboxylase gene expression through an upstream Sp1 site and downstream gastrin responsive elements. J. Biol. Chem. 2004, 279, 8684–8693. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, B.; Harmacek, L.; Long, Z.; Liang, J.; Lukin, K.; Leach, S.M.; O’Connor, B.; Gerber, A.N.; Hagman, J.; et al. The transcription factors GATA2 and microphthalmia-associated transcription factor regulate Hdc gene expression in mast cells and are required for IgE/mast cell-mediated anaphylaxis. J. Allergy Clin. Immunol. 2018, 42, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, J.; Zhao, D.; Guan, X.; Morris, S.C.; Finkelman, F.D.; Huang, H. The Hdc GC box is critical for Hdc gene transcription and histamine-mediated anaphylaxis. J. Allergy Clin. Immunol. 2023, in press. [Google Scholar] [CrossRef]
- Osada, A.; Horikawa, K.; Wakita, Y.; Nakamura, H.; Ukai, M.; Shimura, H.; Jitsuyama, Y.; Suzuki, T. Rosa davurica Pall., a useful Rosa species for functional rose hip production with high content of antioxidants and multiple antioxidant activities in hydrophilic extract. Sci. Hortic. 2022, 291, 110528. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Bazhenova, B.A.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. Originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Three Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Fang, M.; Lee, H.M.; Oh, S.; Zheng, S.; Bellere, A.D.; Kim, M.; Choi, J.; Kim, M.; Yu, D.; Yi, T.H. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Lee, D.Y.; Koh, P.O.; Yang, H.R.; Kang, C.; Kim, E. Rosa davurica Pall. Improves Propionibacterium acnes-Induced Inflammatory Responses in Mouse Ear Edema Model and Suppresses Pro-Inflammatory Chemokine Production via MAPK and NF-κB Pathways in HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.C.; Proud, D.; Lichtenstein, L.M.; MacGlashan, D.W.; Schleimer, R.P., Jr.; Adkinson, N.F.; Kagey-Sobotka, A., Jr.; Schulman, E.S.; Plaut, M. Human lung macrophage-derived histamine-releasing activity is due to IgE-dependent factors. J. Immunol. 1986, 136, 2588–2595. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, A.M.; Newball, H.H.; Dvorak, H.F.; Lichtenstein, L.M. Antigen-induced IgE-mediated degranulation of human basophils. Lab. Investig. 1980, 43, 126–139. [Google Scholar]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, H.; Zarnegar, B.; Westin, A.; Persson, V.; Peuckert, C.; Jonsson, J.; Hallgren, J.; Kullander, K. SLC10A4 regulates IgE-mediated mast cell degranulation in vitro and mast cell-mediated reactions in vivo. Sci. Rep. 2017, 7, 1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.E.; Zhu-Mauldin, X.; Marchase, R.B.; Chatham, J.C. STIM1/Orai1-mediated SOCE: Current perspectives and potential roles in cardiac function and pathology. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H446–H458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroody, F.M.; Naclerio, R.M. Antiallergic effects of H1-receptor antagonists. Allergy 2000, 64, 17–27. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M.; Thirugnanasambantham, K.; Ibrahim, H.I.M. Anti-Allergic Potential of Cinnamaldehyde via the Inhibitory Effect of Histidine Decarboxylase (HDC) Producing Klebsiella pneumonia. Molecules 2020, 25, 5580. [Google Scholar] [CrossRef]
- Do, N.Q.; Zheng, S.; Oh, S.; Nguyen, Q.T.N.; Fang, M.; Kim, M.; Choi, J.; Kim, M.J.; Jeong, J.; Yi, T.H. Anti-Allergic Effects of Myrciaria dubia (Camu-Camu) Fruit Extract by Inhibiting Histamine H1 and H4 Receptors and Histidine Decarboxylase in RBL-2H3 Cells. Antioxidants 2021, 11, 104. [Google Scholar] [CrossRef]












| Primer 1 | Sequence (5′-3′) | |
|---|---|---|
| Rat GAPDH | Sense | TGA TGA CAT CAA GAA GGT GGT GAA G |
| Anti-sense | TCC TTG GAG GCC ATG TAG GCC AT | |
| Rat COX-2 | Sense | ACT CAC TCA GTT TGT TGA GTC ATT C |
| Anti-sense | TTT GAT TAG TAC TGT AGG GTT AAT G | |
| Rat iNOS | Sense | CCT CCT CCA CCC TAC CAA GT |
| Anti-sense | CAC CCA AAG TGC TTC AGT CA | |
| Rat IL-1β | Sense | TGC AGA GTT CCC CAA CTG GTA CAT C |
| Anti-sense | GTG CTG CCT AAT GTC CCC TTG AAT C | |
| Rat IL-6 | Sense | CTG CAA GAG ACT TCC ATC CAG |
| Anti-sense | AGT GGT ATA GAC AGG TCT GTT GG | |
| Rat TNF-α | Sense | TCT CAT CAG TTC TAT GGC CC |
| Anti-sense | GGG AGT AGA CAA GGT ACA AC | |
| Mouse GAPDH | Sense | TGA TGA CAT CAA GAA GGT GGT GAA G |
| Anti-sense | TCC TTG GAG GCC ATG TAG GCC AT | |
| Mouse HDC | Sense | TGA CAA CTT CTC ACT CCG AGG |
| Anti-sense | ACA AGG TTA GCA GCC TCT CG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Oh, S.; Nguyen, Q.T.N.; Kim, M.; Zheng, S.; Fang, M.; Yi, T.-H. Rosa davurica Inhibited Allergic Mediators by Regulating Calcium and Histamine Signaling Pathways. Plants 2023, 12, 1572. https://doi.org/10.3390/plants12071572
Lim S, Oh S, Nguyen QTN, Kim M, Zheng S, Fang M, Yi T-H. Rosa davurica Inhibited Allergic Mediators by Regulating Calcium and Histamine Signaling Pathways. Plants. 2023; 12(7):1572. https://doi.org/10.3390/plants12071572
Chicago/Turabian StyleLim, Seojun, Sarang Oh, Quynh T. N. Nguyen, Myeongju Kim, Shengdao Zheng, Minzhe Fang, and Tae-Hoo Yi. 2023. "Rosa davurica Inhibited Allergic Mediators by Regulating Calcium and Histamine Signaling Pathways" Plants 12, no. 7: 1572. https://doi.org/10.3390/plants12071572