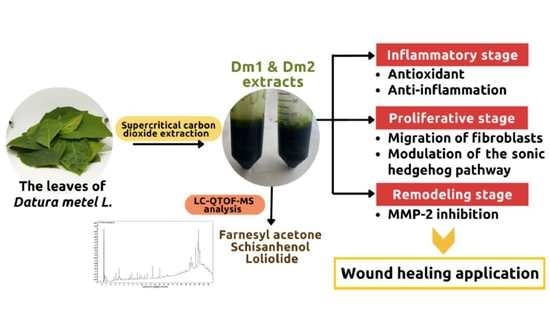
Wound Healing Effect of Supercritical Carbon Dioxide Datura metel L. Leaves Extracts: An In Vitro Study of Anti-Inflammation, Cell Migration, MMP-2 Inhibition, and the Modulation of the Sonic Hedgehog Pathway in Human Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. Supercritical Carbon Dioxide (scCO2) Extraction Conditions and Extraction Yields of Datura metel
2.2. Phytochemical Constituents from Datura metel Extracts
2.3. Cell Viability
2.4. Antioxidant Properties
2.4.1. Antioxidant Activities against DPPH and ABTS Radicals
2.4.2. Cellular Antioxidant Activity
2.5. Anti-Inflammatory Activity
2.6. Migration of Fibroblasts
2.7. Expression Levels of Matrix Metalloproteinase Type 2
2.8. Effects of Datura metel Extracts on Signaling Pathways in Human Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extract Preparation
4.2. Quanlitative Analysis Using LC-QTOF-MS
4.3. Cell Culture
4.4. Cytotoxicity Assay
4.5. In Vitro Antioxidant Assay
4.5.1. Scavenging Capacity Assay
4.5.2. Thiobarbituric Acid-Reactive Substances (TBARS) Method
4.6. Nitric Oxide Assay
4.7. Scratch Assay
4.8. Gelatin Zymography for MMP-2 Activity Analysis
4.9. Semi-Quantitative RT-PCR Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Snafi, A.E. Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium-A review. IOSR J. Pharm. 2017, 7, 43–58. [Google Scholar] [CrossRef]
- Phargarden.Com. Available online: https://apps.phar.ubu.ac.th/phargarden/main.php?action=viewpage&pid=105 (accessed on 25 April 2023).
- Tan, J.Y.; Liu, Y.; Cheng, Y.G.; Sun, Y.P.; Pan, J.; Yang, S.H.; Kuang, H.X.; Yang, B.Y. Anti-inflammatory sesquiterpenoids from the leaves of Datura metel L. Fitoterapia 2020, 142, 104531. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Anisha, S.; Khusro, A.; Essa, M.M.; Chidambaram, S.B.; Qoronfleh, M.W.; Sadhasivam, S.; Sahibzada, M.U.K.; Alghamdi, S.; Almehmadi, M. Anti-pathogenic, anti-diabetic, anti-inflammatory, antioxidant, and wound healing efficacy of Datura metel L. leaves. Arab. J. Chem. 2022, 15, 104112. [Google Scholar] [CrossRef]
- Guo, R.; Liu, Y.; Pan, J.; Guan, W.; Yang, B.Y.; Kuang, H.X. A new sesquiterpenoid with cytotoxic and anti-inflammatory activity from the leaves of Datura metel L. Nat. Prod. Res. 2021, 35, 607–613. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Tan, J.; Sun, Y.; Guan, W.; Jiang, P.; Yang, B.; Kuang, H. Integrated serum metabolomics and network pharmacology approach to reveal the potential mechanisms of withanolides from the leaves of Datura metel L. on psoriasis. J. Pharm. Biomed. Anal. 2020, 186, 113277. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Chong, D.L.; Trinder, S.; Labelle, M.; Rodriguez Justo, M.; Hughes, S.; Holmes, A.M.; Scotton, C.J.; Porter, J.C. Platelet-derived transforming growth factor-β1 promotes keratinocyte proliferation in cutaneous wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 645–649. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Gonzalez, A.C.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.X.; Sun, C.C.; Zhu, Y.T.; Wang, Y.; Wang, T.; Chi, L.S.; Cai, W.H.; Zheng, J.Y.; Zhou, X.; Cong, W.T. Hedgehog signaling contributes to basic fibroblast growth factor-regulated fibroblast migration. Exp. Cell Res. 2017, 355, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E. Phytochemical constitution, anti-inflammation, anti-androgen, and hair growth-promoting potential of shallot (Allium ascalonicum L.) extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Khantham, C.; Ruksiriwanich, W.; Sringarm, K.; Prom-u-thai, C.; Jamjod, S.; Arjin, C.; Muangsanguan, A.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Effects of bioactive composition in Oryza sativa L. cv. KDML105 bran extract on gene expression related to hair cycle in human hair follicle dermal papilla cells. Agronomy 2023, 13, 295. [Google Scholar] [CrossRef]
- Wier, E.M.; Garza, L.A. Through the lens of hair follicle neogenesis, a new focus on mechanisms of skin regeneration after wounding. Semin. Cell Dev. Biol. 2020, 100, 122–129. [Google Scholar] [CrossRef]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Banday, J.A.; Yatoo, G.N.; Hajam, M.A.; Bhat, S.A.; Santhanakrishnan, V.P.; Farozi, A.; Rather, M.A.; Rasool, S. Gas chromatographic-mass spectrometric analysis, antioxidant, antiproliferative and antibacterial activities of the essential oil of Prangos pabularia. Microb. Pathog. 2022, 166, 105540. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Ozer, M.S.; Calli, N.; Popović Djordjević, J. Essential oil composition and antioxidant activity of endemic Marrubium parviflorum subsp. oligodon. Ind. Crop. Prod. 2018, 119, 209–213. [Google Scholar] [CrossRef]
- Heredia, D.; Green, I.; Klaasen, J.; Rahiman, F. Importance and relevance of phytochemicals present in Galenia africana. Scientifica 2022, 2022, 5793436. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Cheng, S.; Li, J.; Xu, X.; Hao, X.; Fan, Y.; Mu, S. Synthesis and biological evaluation of novel schisanhenol derivatives as potential hepatoprotective agents. Eur. J. Med. Chem. 2022, 227, 113919. [Google Scholar] [CrossRef] [PubMed]
- Bend, J.R.; Xia, X.Y.; Chen, D.; Awaysheh, A.; Lo, A.; Rieder, M.J.; Jane Rylett, R. Attenuation of oxidative stress in HEK 293 cells by the TCM constituents schisanhenol, baicalein, resveratrol or crocetin and two defined mixtures. J. Pharm. Pharm. Sci. 2015, 18, 661. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Chen, J.; Mu, Y.; Zhang, H.; Chen, G.; Liu, P.; Liu, W. The effects of inhibiting the activation of hepatic stellate cells by lignan components from the fruits of Schisandra chinensis and the mechanism of schisanhenol. J. Nat. Med. 2020, 74, 513–524. [Google Scholar] [CrossRef]
- Bedane, K.G.; Zühlke, S.; Spiteller, M. Bioactive constituents of Lobostemon fruticosus: Anti-inflammatory properties and quantitative analysis of samples from different places in South Africa. S. Afr. J. Bot. 2020, 131, 174–180. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Heo, S.J.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Sanjeewa, K.K.A.; Lee, K.; Ahn, G. (−)-Loliolide isolated from Sargassum horneri abate UVB-induced oxidative damage in human dermal fibroblasts and subside ECM degradation. Mar. Drugs 2021, 19, 435. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.S.; Kim, S.; Lorz, L.R.; Choi, E.; Lim, H.Y.; Hossain, M.A.; Jang, S.; Choi, Y.I.; Park, K.J. Loliolide presents antiapoptosis and antiscratching effects in human keratinocytes. Int. J. Mol. Sci. 2019, 20, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The effect of comorbidities on wound healing. Surg. Clin. 2020, 100, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous wound healing: An update from physiopathology to current therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Carr, A.L.; Sun, L.; Drewing, A.; Lee, J.; Rao, Z. A novel function of the human oncogene Stil: Regulation of PC12 cell toxic susceptibility through the Shh pathway. Sci. Rep. 2015, 5, 16513. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Rinkevich, Y. Scars or regeneration?—Dermal fibroblasts as drivers of diverse skin wound responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef] [Green Version]
- Kanno, E.; Tanno, H.; Masaki, A.; Sasaki, A.; Sato, N.; Goto, M.; Shisai, M.; Yamaguchi, K.; Takagi, N.; Shoji, M. Defect of interferon γ leads to impaired wound healing through prolonged neutrophilic inflammatory response and enhanced MMP-2 activation. Int. J. Mol. Sci. 2019, 20, 5657. [Google Scholar] [CrossRef] [Green Version]
- Ruksiriwanich, W.; Khantham, C.; Sringarm, K.; Sommano, S.; Jantrawut, P. Depigmented Centella asiatica extraction by pretreated with supercritical carbon dioxide fluid for wound healing application. Processes 2020, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R. In Vitro and in vivo regulation of SRD5A mRNA expression of supercritical carbon dioxide extract from Asparagus racemosus Willd. Root as anti-sebum and pore-minimizing active ingredients. Molecules 2022, 27, 1535. [Google Scholar] [CrossRef]
- Arjin, C.; Hongsibsong, S.; Pringproa, K.; Seel-Audom, M.; Ruksiriwanich, W.; Sutan, K.; Sommano, S.R.; Sringarm, K. Effect of ethanolic Caesalpinia sappan fraction on in vitro antiviral activity against porcine reproductive and respiratory syndrome virus. Vet. Sci. 2021, 8, 106. [Google Scholar] [CrossRef]
- Linsaenkart, P.; Ruksiriwanich, W.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C. Natural melanogenesis inhibitor, antioxidant, and collagen biosynthesis stimulator of phytochemicals in rice bran and husk extracts from purple glutinous rice (Oryza sativa L. cv. Pieisu 1 CMU) for cosmetic application. Plants 2023, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Chiangnoon, R.; Samee, W.; Uttayarat, P.; Jittachai, W.; Ruksiriwanich, W.; Sommano, S.R.; Athikomkulchai, S.; Chittasupho, C. Phytochemical analysis, antioxidant, and wound healing activity of Pluchea indica L. (Less) branch extract nanoparticles. Molecules 2022, 27, 635. [Google Scholar] [CrossRef] [PubMed]









| Experimental Conditions | Dm1 | Dm2 |
|---|---|---|
| Temperature (°C) | 35 | 35 |
| Pressure (bar) | 300 | 500 |
| Co-solvent | 95% Ethanol | 95% Ethanol |
| Yield of Extract (% w/w) | 2.7 ± 0.2 | 3.0 ± 0.1 |
| Name | Molecular Formation | RT (min) | Mass (m/z) | Matching Score (%) | ||
|---|---|---|---|---|---|---|
| Dm1 | Dm2 | |||||
| 1 | Dihydroferuperine | C17H23NO3 | 4.923 | 289.1681 | 99.48 | 99.08 |
| 2 | Farnesyl acetone | C18H30O | 20.831 | 262.2300 | 98.79 | 97.99 |
| 3 | Schisanhenol B | C22H26O6 | 12.443 | 386.1730 | 99.47 | 99.48 |
| 4 | Hyoscine | C17H21NO4 | 4.151 | 303.1472 | 85.42 | 85.27 |
| 5 | Bufotalinin | C24H30O6 | 14.099 | 414.2043 | 99.62 | 99.63 |
| 6 | N-Hexadecanoylpyrrolidine | C20H39NO | 25.231 | 309.3034 | 99.74 | 99.71 |
| 7 | Sphinganine | C16H35NO2 | 10.628 | 273.2669 | 99.77 | 99.83 |
| 8 | Convolamine | C17H23NO4 | 4.213 | 305.1628 | 99.57 | 99.79 |
| 9 | Ambronide | C16H28O | 19.920 | 236.2145 | 98.82 | 98.65 |
| 10 | Moprolol | C13H21NO3 | 4.393 | 239.1523 | 99.73 | 99.73 |
| 11 | Loliolide | C11H16O3 | 6.964 | 196.1098 | 85.58 | 86.28 |
| 12 | 9Z,12E,15E-Octadecatrienoic acid | C18H30O2 | 21.592 | 278.2244 | 84.82 | 82.68 |
| Samples | IC50 for DPPH (mg/mL) | IC50 for ABTS (mg/mL) |
|---|---|---|
| Dm1 | 0.35 ± 0.00 a | 0.47 ± 0.04 a |
| Dm2 | 0.31 ± 0.01 a | 0.36 ± 0.01 a |
| Trolox | 0.02 ± 0.00 b | 0.16 ± 0.00 b |
| Functional Pathway | Genes | Sequences |
|---|---|---|
| Angiogenesis pathway | VEGF | Forward: CTACCTCCACCATGCCAAGT Reverse: GCGAGTCTGTGTTTTTGCAG |
| Wnt/β-catenin signaling | CTNNB1 | Forward: CCCACTAATGTCCAGCGTTT Reverse: AACCAAGCATTTTCACCAGG |
| Sonic hedgehog pathway | SHH | Forward: AAAAGCTGACCCCTTTAGCC Reverse: GCTCCGGTGTTTTCTTCATC |
| SMO | Forward: GAAGTGCCCTTGGTTCGGACA Reverse: CCGCCAGTCAGCCACGAAT | |
| GLI1 | Forward: GCAGGGAGTGCAGCCAATACAG Reverse: GAGCGGCGGCTGACAGTATA | |
| Reference gene | GAPDH | Forward: GGAAGGTGAAGGTCGGAGTC Reverse: CTCAGCCTTGACGGTGCCATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruksiriwanich, W.; Linsaenkart, P.; Muangsanguan, A.; Sringarm, K.; Jantrawut, P.; Arjin, C.; Sommano, S.R.; Phimolsiripol, Y.; Barba, F.J. Wound Healing Effect of Supercritical Carbon Dioxide Datura metel L. Leaves Extracts: An In Vitro Study of Anti-Inflammation, Cell Migration, MMP-2 Inhibition, and the Modulation of the Sonic Hedgehog Pathway in Human Fibroblasts. Plants 2023, 12, 2546. https://doi.org/10.3390/plants12132546
Ruksiriwanich W, Linsaenkart P, Muangsanguan A, Sringarm K, Jantrawut P, Arjin C, Sommano SR, Phimolsiripol Y, Barba FJ. Wound Healing Effect of Supercritical Carbon Dioxide Datura metel L. Leaves Extracts: An In Vitro Study of Anti-Inflammation, Cell Migration, MMP-2 Inhibition, and the Modulation of the Sonic Hedgehog Pathway in Human Fibroblasts. Plants. 2023; 12(13):2546. https://doi.org/10.3390/plants12132546
Chicago/Turabian StyleRuksiriwanich, Warintorn, Pichchapa Linsaenkart, Anurak Muangsanguan, Korawan Sringarm, Pensak Jantrawut, Chaiwat Arjin, Sarana Rose Sommano, Yuthana Phimolsiripol, and Francisco J. Barba. 2023. "Wound Healing Effect of Supercritical Carbon Dioxide Datura metel L. Leaves Extracts: An In Vitro Study of Anti-Inflammation, Cell Migration, MMP-2 Inhibition, and the Modulation of the Sonic Hedgehog Pathway in Human Fibroblasts" Plants 12, no. 13: 2546. https://doi.org/10.3390/plants12132546