Red Mangrove (Rhizophora stylosa Griff.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Prospects
Abstract
:1. Introduction
2. Methodology
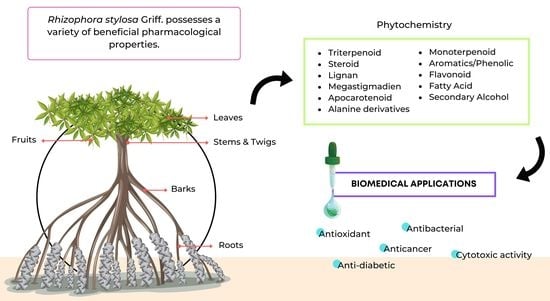
3. Botany
4. Phytochemistry
4.1. Terpenoid
4.2. Flavonoids
4.3. Other Compounds
5. Ethnobotany and Medicinal Uses
6. Biological Activities
6.1. Antibacterial Activity
6.2. Cytotoxic Activity
6.3. Antioxidant Property
6.4. Anti-Diabetic Property
7. Prospects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusmana, C.; Sukristijiono. Mangrove resource uses by local community in Indonesia. J. Nat. Res. Environ. Manag. 2016, 6, 217–224. [Google Scholar] [CrossRef]
- Mitra, S.; Naskar, N.; Chaudhuri, P. A review on potential bioactive phytochemicals for novel therapeutic applications with special emphasis on mangrove species. Phytomedicine Plus 2021, 1, 100107. [Google Scholar] [CrossRef]
- Miranti, D.I.; Ichiura, H.; Ohtani, Y. The bioactive compounds and antioxidant activity of food products of Rhizophora stylosa fruit (coffee and tea mangrove). J. For. Res. 2018, 2018, 2315329. [Google Scholar] [CrossRef]
- Setyawan, A.D.; Ragavan, P.; Basyuni, M.; Sarno, S. Review: Rhizophora mucronata as source of foods and medicines. Bonorowo Wetl. 2019, 9, 42–55. [Google Scholar] [CrossRef]
- Srikanth, S.; Lum, S.K.; Chen, Z. Mangrove root: Adaptations and ecological importance. Trees 2015, 30, 451–465. [Google Scholar] [CrossRef]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity, and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Bandaranayake, W. Bioactivities, bioactive compounds, and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Habeebula, M.; Velraj, M. Potential anti-diabetic mangroves in Kerala, India: A Review. Int. J. Res. Ayuverda Pharm. 2018, 9, 194–198. [Google Scholar]
- Rout, P.; Singh, S.; Kumar, N.; Basak, U.C. Nutritional and antioxidant potential of some selected edible mangrove fruits of Odisha coast. Int. J. Adv. Sci. Res. 2015, 1, 349–355. [Google Scholar] [CrossRef]
- Kainuma, M.; Kezuka, M.; Inoue, T.; Chan, E.W.; Tangah, J. Botany, uses, chemistry and bioactivities of mangrove plants I: Rhizophora stylosa. ISME/GLOMIS Electron. J. 2015, 13, 12–17. [Google Scholar]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.-Y.; Pan, J.-Y.; Yang, M.-H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Sormin, R.B.; Nendissa, D.M.; Mailoa, M.N.; Rieuwpassa, F.; Wenno, M.R. Antibacterial activity of Rhizophora apiculata extract originated from Inner Ambon Bay against selected pathogen bacteria. In Proceedings of the International Conference on Small Islands Community: Momentum of Transformation of Small Islands Community in New Normal Era, Maluku, Indonesia, 12 December 2020; p. 12017. [Google Scholar]
- Li, D.; Li, X.-M.; Wang, B.-G. Pentacyclic triterpenoids from the mangrove plant Rhizophora stylosa. Nat. Prod. Rep. 2008, 22, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Pramudji, P.; Dharmawan, I.W.E. Growth analysis of Rhizophora stylosa Griff. seedlings in mangrove rehabilitation area of Tanjung Pasir, Tangerang. Oseanologi Limnol. Indones. 2016, 1, 91–100. [Google Scholar]
- Setyawan, A.D.; Ulumuddin, Y.I. Species diversity of Rhizophora in Tambelan Islands, Natuna Sea, Indonesia. Biodiversitas 2012, 13, 172–177. [Google Scholar] [CrossRef]
- Chanda, A.; Akhand, A.; Manna, S.; Das, S.; Mukhopadhyay, A.; Das, I.; Hazra, S.; Choudhury, S.B.; Dadhwal, V.K. Mangrove associates versus true mangroves: A comparative analysis of leaf litter decomposition in Sundarban. Wetl. Ecol. Manag. 2015, 24, 293–315. [Google Scholar] [CrossRef]
- Lalitha, P.; Parthiban, A.; Sachithanandam, V.; Purvaja, R.; Ramesh, R. Antibacterial and antioxidant potential of GC-MS analysis of crude ethyl acetate extract from the tropical mangrove plant Avicennia officinalis L. S. Afr. J. Bot. 2021, 142, 149–155. [Google Scholar] [CrossRef]
- Syahidah; Subekti, N. Phytochemical Analysis of Mangrove Leaves (Rhizophora sp.). In Proceedings of the 14th Pacific Rim Bio-Based Composites Symposium, Makassar, Indonesia, 29–31 October 2018; pp. 1–7. [Google Scholar]
- Arbiastutie, Y.; Diba, F.; Masriani, M. Short Communication: Ethnobotanical and ecological studies of medicinal plants in a mangrove forest in Mempawah District, West Kalimantan, Indonesia. Biodiversitas 2021, 22, 3164–3170. [Google Scholar] [CrossRef]
- Gopal, N.; Ekegbu, J.; Kaur, C.P.; Paulraj, P.; P, R.; Bhavya, K.S. Evaluation of antibacterial properties of leaves and barks of Rhizophora stylosa against Gram-Positive and Gram-Negative organisms. J. Pure. Appl. Microbiol. 2019, 13, 957–965. [Google Scholar] [CrossRef]
- Ng, W.L.; Chan, H.T. Survey of Rhizophora stylosa populations in Peninsular Malaysia. ISME/GLOMIS Electron. J. 2012, 10, 4–6. [Google Scholar]
- Duke, N.C. Indo-Wes Pacific stilt mangroves: Rhizophora apiculata, R. mucronata, R. stylosa, R. × annamalai, R. × lamarckii. In Traditional Trees of Pacific Islands; Elevitch, C.R., Ed.; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2006; pp. 641–660. [Google Scholar]
- Juliana, W.W.; Farihah, A.; Akmar, Z.N.; Razali, S.M.; Nuhanim, M. Phenology of Rhizophora species at three Peninsular Malaysia mangrove forests. In Proceedings of the 10th International Annual Symposium (UMTAS 2011), Kuala Terengganu, Malaysia, 11–13 July 2011; Universiti Malaysia Terengganu: Kuala Terengganu, Malaysia, 2011; pp. 12–17. [Google Scholar]
- Coupland, G.T.; Paling, E.I.; McGuinness, K.A. Floral abortion and pollination in four species of tropical mangroves from northern Australia. Aquat. Bot. 2006, 84, 151–157. [Google Scholar] [CrossRef]
- Duke, N.C.; Bunt, J.S.; Williams, W.T. Observations on the floral and vegetative phenologies of north-eastern Australian mangroves. Aus. J. Bot. 1984, 32, 87–99. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Sharma, S.; Kamara, M.; Hagihara, A. Phenological traits of the mangrove Rhizophora stylosa Griff. at the northern limit of its biogeographical distribution. Wetl. Ecol. Manag. 2013, 21, 277–288. [Google Scholar] [CrossRef]
- Paryanto, P.; Pranolo, S.H.; Susanti, A.D.; Dewi, K.R.; Rossari, M. Chemical Structure of Mangrove Species Rhizophora stylosa as Natural Dyes. METANA 2020, 16, 33–38. [Google Scholar] [CrossRef]
- Wafar, S.; Untawale, A.G.; Wafar, M. Litter fall and energy flux in a mangrove ecosystem. Estuar. Coast. Shelf Sci. 1997, 44, 111–124. [Google Scholar] [CrossRef]
- Ochieng, C.A.; Erftemeijer, P.L. Phenology, litterfall and nutrient resorption in Avicennia marina (Forssk.) Vierh in Gazi Bay, Kenya. Trees 2002, 16, 167–171. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Sharma, S.; Hoque, A.T.M.R.; Hagihara, A. Litterfall of three subtropical mangrove species in the family Rhizophoraceae. J. Oceanogr. 2012, 68, 841–850. [Google Scholar] [CrossRef]
- Yang, X.-H.; Li, H.-B.; Chen, H.; Li, P.; Ye, B.-P. Chemical constituents in the leave of Rhizophora stylosa L. and their biological activities. Acta Pharm. Sin. 2008, 43, 974–978. [Google Scholar]
- Huong, P.T.; Diep, C.N.; Thanh, N.V.; Tu, V.A.; Hanh, T.H.; Cuong, N.T.; Thao, N.P.; Cuong, N.X.; Thao, D.T.; Thai, T.H.; et al. A new cycloartane glucoside from Rhizophora stylosa. Nat. Prod. Comm. 2014, 9, 1255–1257. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y.; Takaso, T.; Tobe, H. Floral scent chemistry of mangrove plants. J. Plant. Res. 2002, 115, 47–53. [Google Scholar] [CrossRef]
- Li, D.-L.; Li, X.-M.; Peng, Z.-Y.; Wang, B.-G. Flavanol derivates from Rhizophora stylosa and their DPPH radical scavenging activity. Molecules 2007, 12, 1163–1169. [Google Scholar] [CrossRef]
- Takara, K.; Kuniyoshi, A.; Wada, K.; Kinjyo, K.; Iwasaki, H. Antioxidative flavan-3-ol glycosides from Stems of Rhizophora stylosa. Biosci. Biotechnol. Biochem. 2008, 72, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Ncube, N.; Afolayan, A.; Okoh, A. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Abubakar, S.; Kadir, M.A.; Wibowo, E.S.; Akbar, N. Benefits of mangrove for pharmacitic inventory in Mamuya Village, East Galela District, East Halmahera Regency (Ethnopharmacological Review). J. Enggano 2019, 4, 12–25. [Google Scholar]
- Lalitha, P.; Sachithanandam, V.; Swarnakumar, N.S.; Sridhar, R. Review on Anti-inflammatory Properties of Mangrove plants. Asian J. Pharm. Res. 2019, 9, 273–288. [Google Scholar] [CrossRef]
- Nanuru, E.W.; Dewi, L.; Wibowo, P. Effect of asiatic mangrove (Rhizophora mucronata) leaves extract as analgesic in male albino DDW mice (Mus musculus L.) induced by 0.7% acetic acid. Med. Health Sci. J. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Ramanathan, T.; Hariharan, B.; Ganesan, K. Antidiabetic activity of coastal mangrove leaves of Rhizophora mucronata. Plant Arch. 2008, 8, 931–933. [Google Scholar]
- Alikuhni, N.M.; Kandasamy, K.; Manoharan, C.; Subramanian, M. Insulin-like antigen of mangrove leaves and its anti-diabetic activity in alloxan-induced diabetic rats. Nat. Prod. Res. 2012, 26, 1161–1166. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, Z.; Zhang, W.; Xu, J. Evaluation of the antimicrobial and cytotoxic potential of endophytic fungi extracts from mangrove plants Rhizophora stylosa and R. mucronata. Sci. Rep. 2022, 12, 2733. [Google Scholar] [CrossRef]
- Mouafi, F.E.; Abdel-Aziz, S.M.; Bashir, A.A.; Fyiad, A.A. Phytochemical analysis and antimicrobial activity of mangrove leaves (Avicenna marina and Rhizophora stylosa) against some pathogens. World Appl. Sci. J. 2014, 29, 547–554. [Google Scholar]
- Usman, M.M.A.; Erika, F.; Nurdin, M.; Kuncoro, H. Antidiabetic activity of leaft extract from three types of mangrove originating from Sambera Coastal Region Indonesia. Res. J. Pharm. Technol. 2019, 12, 1707–1712. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Park, H.-S.; Lee, T.-K. Phenol content, antioxidant and tyrosinase inhibitory activity of mangrove plants in Micronesia. Asian Pac. J. Trop. Med. 2014, 7, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Vikram, P.; Chiruvella, K.K.; Ripain, I.H.A.; Arifullah, M. A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.). Asian Pac. J. Trop. Biomed. 2014, 4, 430–435. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoaty, M.A.; Ibrahim, M.A.; Ahmed, N.S.; Abdelaziz, M.A. Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J. Clin. Biochem. 2010, 25, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Maddaluri, S.; Rao, K.B.; Sitaram, B. In vitro evaluation of antibacterial activity of five indigenous plants extract against five bacterial pathogens of human. Int. J. Pharm. Pharm. Sci. 2013, 5, 679–684. [Google Scholar]
- Efendi, A.; Halid, I.; Ustiawaty, J. Effect of Rhizophora sp. mangrove leaf extract on mice blood glucose levels. In Proceedings of the 3rd International Conference on Bioscience and Biotechnology, Lombok, Indonesia, 12–14 October 2020. [Google Scholar]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Willian, N.; Syukri, S.; Zulhadjri, Z.; Pardi, H.; Arief, S. Marine plant mediated green synthesis of silver nanoparticle using mangrove Rhizophora stylosa: Effect of variable process and their antibacterial activity. F1000Research 2022, 10, 768. [Google Scholar] [CrossRef]
- Vasanth, K.; Ilango, K.; Mohankumar, R.; Agrawal, A.; Dubey, G. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef]
- Ali, A.; Akhtar, N.; Khan, B.; Khan, H.; Rasul, A.; Khalid, N.; Waseem, K. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plants Res. 2012, 6, 1492–1496. [Google Scholar]
- Hembram, K.C.; Kumar, R.; Kandha, L.; Parhi, P.K.; Kundu, C.N.; Bindhani, B.K. Therapeutic prospective of plant-induced silver nanoparticles: Application as antimicrobial and anticancer agent. Artif. Cells Nanomed. Biotechnol. 2018, 46, S38–S51. [Google Scholar] [CrossRef]
- Srirangam, G.; Rao, K.P. Synthesis and characterization of silver nanoparticles from the leaf extract of Malachra capitata (L.). RASAYAN J. Chem. 2017, 10, 46–53. [Google Scholar]
- Willian, N.; Syukri, Z.; Labanni, A.; Arief, S. Bio-Friendly synthesis of silver nanoparticles using mangrove Rhizophora stylosa leaf aqueous extract and its antibacterial and antioxidant activity. RASAYAN J. Chem. 2020, 13, 1478–1485. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Zengin, G.; Mahomoodally, M.F. Biotechnological applications of mangrove plants and their isolated compounds in medicine-a mechanistic overview. Crit. Rev. Biotechnol. 2022, 43, 393–414. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Singh, H.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J. Nanomater. 2015, 2015, 4. [Google Scholar] [CrossRef]
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Balavandy, S.K.; Shameli, K.; Biak, D.R.; Abidin, Z.Z. Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Chem. Cent. J. 2014, 8, 11. [Google Scholar] [CrossRef]





| Phytochemical Classes | Compounds | References |
|---|---|---|
| Triterpenoid | taraxerone (1) | [31] |
| taraxerol (2) | [13,31] | |
| careaborin (3) | [13,31] | |
| rhizostyloide (4) | [32] | |
| 3β-O-(E)-coumaroyl-15α-hydroxy-β-amyrin (5) | [13] | |
| 15α-hydroxy-β-amyrin (6) | [13] | |
| 3β-taraxerol formate (7) | [13] | |
| 3β-taraxerol acetate (8) | [13] | |
| 3β-O-(Z) coumaroyl-taraxerol (9) | [13] | |
| Monoterpenoid | linalool (10) | [33] |
| eugenol (11) | [33] | |
| Flavonoid | astilbin (12) | [31] |
| rutin (13) | [31] | |
| kaempferol 3-rutinoside (14) | [32] | |
| quercetin-3-O-galactopyranoside (15) | [3] | |
| procyanidin (16) | [3] | |
| prodelphinidin (17) | [3] | |
| 3,7-O-diacetyl (−)-epicatechin (18) | [34] | |
| (−)-epicatechin (19) | [34] | |
| 3-O-acetyl (−)-epicatechin (20) | [34] | |
| (+)-afzelechin (21) | [34] | |
| (+)-catechin (22) | [34] | |
| 3,3′,4′,5,7-O-pentaacetyl-(−)-epicatechin (23) | [34] | |
| proanthocyanidin B2 (24) | [34] | |
| cinchonain Ib (25) | [35] | |
| cinchonain IIa (26) | [35] | |
| cinchonain IIb (27) | [35] | |
| (+)-catechin 3-O-α-L-rhamnoside (28) | [35] | |
| cichnonain Ia (29) | [35] | |
| glabaroside A (30) | [35] | |
| glabaroside B (31) | [35] | |
| Steroid | β-sitosterol (32) | [31] |
| β-daucosterol (33) | [31] | |
| Lignan | (7S,8R)-3,3′,5-trimethoxy-4′,7-epoxy-8,5′-neolignan-4,9,9′-triol (34) | [32] |
| (7S,8R)-3,3′-dimethoxy-4′,7-epoxy-8,5′-neolignan-4,9,9′-triol (35) | [32] | |
| (+)-isolariciresinol (36) | [32] | |
| polystachyol (37) | [32] | |
| (+)-pinoresinol (38) | [32] | |
| Megastigmadien | (6S,7E,9R)-6,9-dihydroxy-4,7-megastigmadien-3-one 9-O-[α-L-arabinopyranosyl-(l→6)-β-D-glucopyranoside] (39) | [32] |
| Apocarotenoid | Blumenol A (40) | [32] |
| Alanine derivatives | N,N-dimethyl-L-alanine (41) | [3] |
| Aromatics/Phenolic | 1,2-dimethoxybenzene (42) | [33] |
| isovanilic acid (43) | [31] | |
| protocatechuic acid (44) | [31] | |
| Fatty Acid | dodecanoic acid (45) | [3] |
| Secondary Alcohol | 2,3-butanediol (46) | [33] |
| Part of Plant | Extracts Used | Phytochemical Detected | Therapeutic Application | References |
|---|---|---|---|---|
| Leaves | EA | Flavonoids, Alkaloids, Terpenoids, Steroids, Cardiac glycosides, and Tannin | Antibacterial | [43] |
| NI | Taraxerol | Cytotoxicity | [31] | |
| Cis-careaborin | Cytotoxicity | |||
| Me | Glucoside Rhizostyloside | Cytotoxicity | [32] | |
| E | Alkaloids, Flavonoids, Steroids, Terpenoids, Phenolic, Tannin, and Saponins | Anti-diabetic | [44] | |
| Me Fractions | Procyanidin | Antioxidant | [3] | |
| Stem and Twigs | NI | Proanthocyanidin | Antioxidant | [34] |
| Me | Phenolic compounds | Antioxidant | [45] | |
| Barks | CE | NI | Antibacterial | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalasuba, K.; Miranti, M.; Rahayuningsih, S.R.; Safriansyah, W.; Syamsuri, R.R.P.; Farabi, K.; Oktavia, D.; Alhasnawi, A.N.; Doni, F. Red Mangrove (Rhizophora stylosa Griff.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Prospects. Plants 2023, 12, 2196. https://doi.org/10.3390/plants12112196
Kalasuba K, Miranti M, Rahayuningsih SR, Safriansyah W, Syamsuri RRP, Farabi K, Oktavia D, Alhasnawi AN, Doni F. Red Mangrove (Rhizophora stylosa Griff.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Prospects. Plants. 2023; 12(11):2196. https://doi.org/10.3390/plants12112196
Chicago/Turabian StyleKalasuba, Karina, Mia Miranti, Sri Rejeki Rahayuningsih, Wahyu Safriansyah, Rizky Riscahya Pratama Syamsuri, Kindi Farabi, Dina Oktavia, Arshad Naji Alhasnawi, and Febri Doni. 2023. "Red Mangrove (Rhizophora stylosa Griff.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Prospects" Plants 12, no. 11: 2196. https://doi.org/10.3390/plants12112196