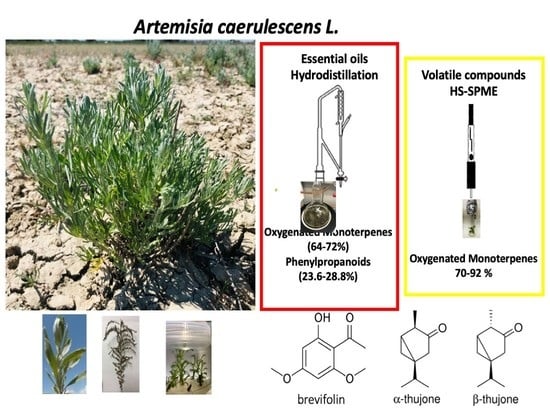
Halophyte Artemisia caerulescens L.: Metabolites from In Vitro Shoots and Wild Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Shoot Proliferation
2.2. Biochemical Analysis
2.3. Phytochemical Investigation
2.3.1. Headspace Analysis
2.3.2. Essential Oil Hydrodistillation
3. Materials and Methods
3.1. Plant Materials
3.2. In Vitro Shoot Proliferation
3.3. Biochemical Analysis
3.3.1. Pigment, Polyphenol, and Flavonoid Extraction and Determination
3.3.2. Antioxidant Activity
3.4. Phytochemical Investigation
3.4.1. HS-SPME Analysis
3.4.2. Essential Oil Hydrodistillation
3.4.3. Gas Chromatography—Mass Spectrometry Analyses
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, M.I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Pereira, C.; Moreira, P.; Salgueiro, L.; Figueirinha, A. Chemical characterization and bioactive potential of Artemisia campestris L. subsp. maritima (DC) Arcang. essential oil and hydrodistillation residual water. J. Ethnopharmacol. 2021, 276, 114146. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive Compounds and Health Benefits of Artemisia Species. Nat. Prod. Commun. 2019, 14, 1934578X1985035. [Google Scholar] [CrossRef] [Green Version]
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Taak, P. The Artemisia Genus: A Review on Traditional Uses, Phytochemical Constituents, Pharmacological Properties and Germplasm Conservation. J. Glycom. Lipidom. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ciccarelli, D.; Giovanelli, S.; Pistelli, L.; Flamini, G.; Cervelli, C.; Mancianti, F.; Nardoni, S.; Bertelloni, F.; Ebani, V.V. Antimicrobial Activity and Composition of Five Rosmarinus (Now Salvia spp. and Varieties) Essential Oils. Antibiotics 2021, 10, 91090. [Google Scholar] [CrossRef]
- Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A. Biological Importance of Essential Oils. In Essential Oils-Oils of Nature; IntechOpen: London, UK, 2020; pp. 1–14. [Google Scholar] [CrossRef] [Green Version]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [Green Version]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [Green Version]
- Rozentsvet, O.A.; Nesterov, V.N.; Bogdanova, E.S.; Tabalenkova, G.N.; Zakhozhiy, I.G.; Popov, A.V. Effect of Saline Soils on the Functional State of Species of the Genus Artemisia. Biol. Bull. 2019, 46, 294–301. [Google Scholar] [CrossRef]
- Lombardi, T.; Bedini, S. Seed Germination Strategies of Mediterranean Halophytes Under Saline Condition. In Handbook of Halophytes; 2021; pp. 1685–1703. [Google Scholar] [CrossRef]
- Sonjak, S.; Udovič, M.; Wraber, T.; Likar, M.; Regvar, M. Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Sečovlje salterns. Soil Biol. Biochem. 2009, 41, 1847–1856. [Google Scholar] [CrossRef]
- Biondi, E.; Burrascano, S.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Galdenzi, D.; Gigante, D.; Lasen, C.; Spampinato, G.; Venanzoni, R.; et al. Diagnosis and syntaxonomic interpretation of Annex I Habitats (Dir. 92/43/EEC) in Italy at the alliance level. Plant Sociol. 2012, 49, 5–37. [Google Scholar] [CrossRef]
- Sciandrello, S.; Tomaselli, V. Coastal salt-marshes plant communities of the Salicornietea fruticosae class in Apulia (Italy). Biologia 2014, 69, 53–69. [Google Scholar] [CrossRef]
- Lombardi, T.; Bedini, S.; Bertacchi, A. Germination ecology of the aromatic halophyte Artemisia caerulescens L.: Influence of abiotic factors and seed after-ripening time. Folia Geobot. 2019, 54, 115–124. [Google Scholar] [CrossRef]
- Borzabad, R.K.; Sudarshana, M.S.; Niranjan, M.H. In Vitro Plant Regeneration from Leaf Explants of Artemisia vulgaris L.—A Medicinal Herb. Mod. Appl. Sci. 2010, 4, 130–134. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Govindaraj, S.; Kumari, B.D.R.; Cioni, P.L.; Flamini, G. Mass propagation and essential oil analysis of Artemisia vulgaris. J. Biosci. Bioeng. 2008, 105, 176–183. [Google Scholar] [CrossRef]
- Clemente, M.; Contreras, P.; Susín, J.; Pliego-Alfaro, F. Micropropagation of Artemisia granatensis. HortScience 1991, 26, 420. [Google Scholar] [CrossRef] [Green Version]
- Zayova, E.G.; Nedev, T.A.; Petrova, D.H.; Zhiponova, M.K.; Chaneva, G.T. Efficient protocol for mass micropropagation of Artemisia annua L. GSC Biol. Pharm. Sci. 2018, 5, 59–68. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Manokari, M. Efficient In Vitro Propagation by Ex Vitro Rooting Methods of Artemisia absinthium L., an Ethnobotanically Important Plant. Chin. J. Biol. 2015, 2015, 273405. [Google Scholar] [CrossRef] [Green Version]
- Koleva, P.; Wolfram, E.; Pedrussio, S.; Raynova, Y.; Evstatieva, L.; Danova, K. In vitro culture development and polyphenolics production of Artemisia alba Turra. J. Biosci. Biotechnol. 2015, 2, 131–136. [Google Scholar]
- Petrova, N.; Koleva, P.; Velikova, V.; Tsonev, T.; Andreeva, T.; Taneva, S.; Krumova, S.; Danova, K. Relations between photosynthetic performance and polyphenolics productivity of Artemisia alba Turra in in vitro tissue cultures. Int. J. Bioautomation 2018, 22, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Sharifivash, R.; Shokrpour, M. Physico-Chemical Evaluation of Some Wormwood (Artemisia absinthium L.) Ecotypes Under Salt Stress Condition. J. Plant Physiol. Breed. 2017, 7, 81–90. [Google Scholar]
- Ghanem, A.-M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 61100. [Google Scholar] [CrossRef]
- Lee, Y.J.; Thiruvengadam, M.; Chung, I.M.; Nagella, P. Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust. J. Crop Sci. 2013, 7, 1921–1926. [Google Scholar]
- Pieracci, Y.; Pistelli, L.; Lari, M.; Iannone, M.; Marianelli, A.; Ascrizzi, R.; Pistelli, L.; Flamini, G. Hibiscus rosa-sinensis as Flavoring Agent for Alcoholic Beverages. Appl. Sci. 2021, 11, 19864. [Google Scholar] [CrossRef]
- Rao, A.; Rao, L. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell. Tissue Organ. Cult. 2015, 122, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Abbasi, B.H.; Ahmad, N.; Khan, H.; Ali, G.S. Strategies to enhance biologically active-secondary metabolites in cell cultures of Artemisia–current trends. Crit. Rev. Biotechnol. 2017, 37, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Uncini-Manganelli, R.E.; Tomei, P.E. Constituents of the Essential Oil of Artemisia coerulescens L. var. palmata Lam. J. Essent. Oil Res. 2001, 13, 125–127. [Google Scholar] [CrossRef]
- Reale, S.; Pace, L.; D’Archivio, A.A.; De Angelis, F.; Marcozzi, G. Volatiles fingerprint of Artemisia umbelliformis subsp. eriantha by headspace-solid phase microextraction GC–MS. Nat. Prod. Res. 2014, 28, 61–66. [Google Scholar] [CrossRef]
- Li, N.; Mao, Y.; Zhang, X. Separation and Identification of Volatile Constituents in Artemisia argyi Flowers by GC-MS with SPME and Steam Distillation. J. Chromatogr. Sci. 2008, 46, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Demirci, B.; Demirci, F.; Başer, K.H.C. Headspace-SPME and hydrodistillation of two fragrant Artemisia sp. Flavour Fragr. J. 2005, 20, 395–398. [Google Scholar] [CrossRef]
- Karadağ, M.; Koyuncu, M.; Atalar, M.N.; Aras, A. SPME/GC-MS analysis of Artemisia campestris subsp. glutinosa, Lavandula angustifolia Mill., and Zingiber officinale volatiles. Erzincan Üniversitesi Fen Bilim. Enstitüsü Derg. 2021, 14, 41–49. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectroscopy; Stream, C., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- National Institute of Standards and Technology. NIST NIST/EPA/NIH Mass Spectral Library; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/ (accessed on 25 November 2021).
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 25 November 2021).
- Zámboriné Németh, É.; Thi Nguyen, H. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Belhattab, R.; Amor, L.; Barroso, J.G.; Pedro, L.G.; Cristina Figueiredo, A. Essential oil from Artemisia herba-alba Asso grown wild in Algeria: Variability assessment and comparison with an updated literature survey. Arab. J. Chem. 2014, 7, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Nikbakht, M.R.; Sharifi, S.; Emami, S.A.; Khodaie, L. Chemical composition and antiprolifrative activity of Artemisia persica Boiss. and Artemisia turcomanica Gand. essential oils. Res. Pharm. Sci. 2014, 9, 155–163. [Google Scholar]
- Portal to the Flora of Italy. 2019. Available online: http://dryades.units.it/floritaly/index.php (accessed on 5 December 2021).
- Peruzzi, L.; Bedini, G. Wikiplantbase #Toscana v2.1. Available online: http://bot.biologia.unipi.it/wpb/toscana/index.html (accessed on 5 December 2021).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Szollosi, R.; Szollosi Varga, I. Total antioxidant power in some species of Labiatae (Adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA; London, UK; Sydney, Australia; Toronto, ON, Canada; San Francisco, CA, USA, 1982; Volume 26. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons, Inc.: New York, NY, USA, 1976; ISBN 047015019X. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]



| Mother Plant | Number of Explants | Contaminated Explants (%) | Developed Shoots (%) | Number of Shoots/Explant | Shoots Length (cm) |
|---|---|---|---|---|---|
| M1 (MS0) | 18 | 32 | 88.9 | 1 | 2.67 ± 1.7 |
| M2 (MS0) | 10 | 0 | 100 | 1 | 3.5 ±1.6 |
| M3 (MS0-BA) | 20 | 90 | 0 | 0 | 0 |
| M4(MS-BA 2 µM) | 10 | 10 | 3 | 3 | 1.2 ± 0.4 |
| M5(MS-BA 2 µM) | 10 | 10 | 5 | 5 | 1.5 ± 0.5 |
| MS0 | 10 | 0 | 100 | 2.1 ± 1.28 C | 3.18 ± 0.26 A |
| MS-BA 1 µM | 10 | 0 | 100 | 8.1 ± 1.63 A | 1.12 ± 0.14 C |
| MS-BA 2 µM | 10 | 0 | 100 | 5.4 ± 1.08 B | 1.5 ± 0.12 B |
| MS-BA 4 µM | 10 | 0 | 100 | 4.6 ± 1.27 B | 0.76 ± 0.11 D |
| Wild Plants | In Vitro Shoots | ||||
|---|---|---|---|---|---|
| Leaves | Young Inflorescences (Blossom) | Ripe Inflorescences | Shoots (MS0) | Shoots (MS-BA 2 µM) | |
| Chlorophyll a (Chl a, µg g−1 FW) | 930.03 ± 12.19 A | 376.99 ± 0.23 BC | 231.31 ± 4.07 C | 208.9 ± 2.76 C | 448.25 ± 3.76 B |
| Chlorophyll b (Chl b, µg g−1 FW) | 182.27 ± 8.36 A | 114.78 ± 0.54 B | 68.71 ± 0.78 B | 66.6. ± 0.68 B | 132.79 ± 3.76 AB |
| Total Chlorophyll (Tchl, 284.9 µg g−1 FW) | 1112.3 ± 20.15 A | 491.76 ± 0.30 BC | 300.02 ± 4.51 C | 275.51 ± 3.44 C | 581.04 ± 14.54 B |
| Total carotenoids (Tcar, µg g−1 FW) | 294.9 ± 2.01 A | 134.39 ± 0.37 B | 91.47 ± 1.61 B | 60.2 ± 0.78 B | 124.11 ± 3.10 B |
| Total Anthocyanins (TA, mg ME g−1 FW) | nd. | 7.95 ± 0.51 AB | 20.47 ± 0.89 A | 1.03 ± 0.05 B | 0.57 ± 0.05 B |
| Total Polyphenols (TP, mg GAE g−1 FW) | 4.62 ± 0.13 B | 17.15 ± 0.12 A | 18.42 ± 0.24 A | 0.71 ± 0.01 B | 0.63 ± 0.05 B |
| Radical scavenging DPPH-assay (µmol TEAC g−1 FW) | 12.25 ± 0.55 B | 82.15 ± 4.80 A | 71.16 ± 2.94 A | 2.53 ± 0.10 B | 1.59 ± 0.16 B |
| Antioxidant activity FRAP assay (mmol Fe2+ g−1 FW) | 24.35 ± 0.86 B | 120.34 ± 3.64 A | 102.3 ± 1.53 AB | 4.91 ± 0.06 B | 4.30 ± 0.3 B |
| Compounds | l.r.i. 1 | l.r.i. 2 | Class | Relative Abundance ± Standar Deviation (%) | |||
|---|---|---|---|---|---|---|---|
| Leaves | Young Inflorescences (Blossom) | Ripe Inflorescences | In Vitro Shoots | ||||
| ethyl 2-methylbutyrate | 850 | 850 | nt | - 3 | 0.1 ± 0.00 | - | - |
| (E)-salvene | 867 | 867 | nt | - | - | 0.4 ± 0.15 | - |
| ethyl isovalerate | 859 | 858 | nt | - | 1.9 ± 0.22 | - | - |
| tricyclene | 922 | 927 | mh | - | 0.3 ± 0.00 | - | |
| α-thujene | 926 | 1102 | mh | 0.1 ± 0.07 | 0.6 ± 0.11 | 0.2 ± 0.06 | - |
| α-pinene | 933 | 939 | mh | 0.9 ± 0.18 | 3.2 ± 0.05 | 0.3 ± 0.09 | - |
| camphene | 948 | 954 | mh | 0.6 ± 0.09 | 0.8 ± 0.04 | - | - |
| sabinene | 973 | 975 | mh | 5.2 ± 0.82 | 16.1 ± 1.06 | 6.5 ± 2.04 | - |
| β-pinene | 977 | 979 | mh | 0.1 ± 0.02 | 0.6 ± 0.03 | 0.2 ± 0.08 | - |
| myrcene | 991 | 991 | mh | 0.2 ± 0.06 | 0.3 ± 0.02 | - | 0.7 ± 0.38 |
| 2-methylbutyl isobutyrate | 1016 | 1017 | nt | - | 0.3 ± 0.03 | 0.2 ± 0.02 | - |
| α-terpinene | 1017 | 1017 | mh | 0.1 ± 0.02 | 0.2 ± 0.01 | 0.1 ± 0.01 | - |
| p-cymene | 1025 | 1025 | mh | 1.4 ± 0.16 | 0.6 ± 0.03 | 1.7 ± 0.25 | - |
| limonene | 1029 | 1029 | mh | 0.3 ± 0.05 | 0.9 ± 0.17 | 0.2 ± 0.05 | - |
| 1,8-cineole | 1031 | 1031 | om | 1.2 ± 0.04 | 1.2 ± 0.06 | 1.2 ± 0.14 | 0.8 ± 0.22 |
| γ-terpinene | 1058 | 1060 | mh | 0.3 ± 0.02 | 0.3 ± 0.00 | 0.6 ± 0.10 | - |
| α-thujone | 1107 | 1102 | om | 67.9 ± 1.56 | 45.4 ± 0.04 | 78.1 ± 2.56 | 83.2 ± 1.41 |
| β-thujone | 1117 | 1114 | om | 8.0 ± 0.64 | 11.2 ± 1.80 | 9.7 ± 0.28 | 7.6 ± 0.16 |
| chrysanthenone | 1126 | 1128 | om | 2.1 ± 0.43 | 2.6 ± 0.54 | - | - |
| trans-pinocarveol | 1139 | 1139 | om | 0.2 ± 0.03 | - | - | - |
| (Z)-tagetenone | 1231 | 1229 | om | - | 0.6 ± 0.02 | - | - |
| (E)-tagetenone | 1240 | 1238 | om | 0.3 ± 0.02 | - | - | - |
| isopiperitenone | 1271 | 1272 * | om | 0.7 ± 0.02 | - | - | - |
| perilla aldehyde | 1273 | 1272 | om | - | 0.5 ± 0.30 | - | - |
| (E,E)-2,4-decadienal | 1316 | 1317 | nt | 0.2 ± 0.03 | - | - | - |
| cyclosativene | 1367 | 1371 | sh | - | - | - | 1.2 ± 0.26 |
| α-copaene | 1376 | 1377 | sh | 0.4 ± 0.06 | - | - | 1.7 ± 0.10 |
| β-elemene | 1392 | 1391 | sh | - | - | 0.6 ± 0.11 | - |
| 2-ethylidene-6-methyl-3,5-heptadienal | 1395 | 1395 | om | 6.4 ± 1.01 | - | - | - |
| cyperene | 1399 | 1399 | sh | 2.0 ± 0.68 | |||
| (E)-β-farnesene | 1458 | 1457 | sh | 1.3 ± 0.17 | |||
| germacrene D | 1481 | 1485 | sh | 0.8 ± 0.20 | |||
| β-selinene | 1486 | 1490 | sh | 0.3 ± 0.02 | - | - | - |
| T-cadinol | 1641 | 1640 | os | 0.7 ± 0.10 | |||
| brevifolin | 1669 | 1675 * | pp | 0.2 ± 0.03 | - | - | - |
| Total identified (%) | 97.3 ± 0.06 | 100.0 ± 0.01 | 100.0 ± 0.01 | 100.0 ± 0.01 | |||
| Chemical Classes | Leaves | Young Inflorescences (Blossom) | Ripe Inflorescences | In Vitro Shoots | |||
| Monoterpene hydrocarbons (mh) | 9.3 ± 0.95 B | 27.2 ± 0.13 A | 9.8 ± 2.66 B | 0.7 ± 0.38 C | |||
| Oxygenated monoterpenes (om) | 87.0 ± 0.97 B | 70.2 ± 0.34 C | 89.1 ± 2.72 AB | 91.6 ± 1.35 A | |||
| Sesquiterpene hydrocarbons (sh) | 0.6 ± 0.08 B | - B | 0.6 ± 0.11 B | 7.0 ± 0.88 A | |||
| Oxygenated sesquiterpenes (os) | - B | - B | - B | 0.7 ± 0.10 A | |||
| Phenylpropanoids (pp) | 0.2 ± 0.03 | - | - | - | |||
| Other non-terpene derivatives (nt) | 0.2 ± 0.03 C | 2.6 ± 0.20 A | 0.5 ± 0.17 B | - C | |||
| Compounds | l.r.i. 1 | l.r.i. 2 | Class | Relative Abundance (%) ± SD | ||
|---|---|---|---|---|---|---|
| Leaves | Young Inflorescences (Blossom) | Ripe Inflorescences | ||||
| ethyl isovalerate | 859 | 858 | nt | - 3 | 0.1 ± 0.03 | - |
| α-pinene | 933 | 939 | mh | - | 0.2 ± 0.03 | 0.1 ± 0.00 |
| sabinene | 973 | 975 | mh | 0.7 ± 0.18 | 1.3 ± 0.01 | 1.2 ± 0.02 |
| p-cymene | 1025 | 1025 | mh | 0.2 ± 0.04 | - | 0.3 ± 0.03 |
| 1,8-cineole | 1031 | 1031 | om | 0.3 ± 0.01 | 0.3 ± 0.02 | 0.3 ± 0.01 |
| γ-terpinene | 1058 | 1060 | mh | - | 0.1 ± 0.01 | 0.1 ± 0.01 |
| cis-sabinene hydrate | 1066 | 1070 | om | 0.3 ± 0.04 | 0.3 ± 0.02 | 0.4 ± 0.05 |
| trans-sabinene hydrate | 1098 | 1098 | om | 0.1 ± 0.03 | 0.2 ± 0.01 | 0.5 ± 0.04 |
| α-thujone | 1107 | 1102 | om | 51.5 ± 0.03 | 39.7 ± 1.81 | 41.7 ± 0.78 |
| filifolone | 1108 | 1109 | om | 1.6 ± 0.54 | - | - |
| β-thujone | 1117 | 1114 | om | 9.3 ± 1.45 | 12.0 ± 0.07 | 9.9 ± 0.27 |
| dehydrosabinaketone | 1119 | 1121 | om | - | 0.2 ± 0.05 | - |
| chrysanthenone | 1126 | 1128 * | om | 9.5 ± 1.24 | 8.1 ± 1.23 | 6.6 ± 0.02 |
| trans-pinocarveol | 1139 | 1139 | om | 0.5 ± 0.05 | 0.4 ± 0.03 | 1.2 ± 0.07 |
| pinocarvone | 1163 | 1165 | om | 0.3 ± 0.04 | 0.2 ± 0.02 | 1.0 ± 0.01 |
| 4-terpineol | 1177 | 1177 | om | 0.4 ± 0.08 | 0.4 ± 0.00 | 0.3 ± 0.00 |
| myrtenal | 1194 | 1196 | om | 0.1 ± 0.02 | 0.1 ± 0.00 | - |
| piperitone | 1254 | 1253 | om | 0.2 ± 0.05 | - | - |
| cis-chrysanthenyl acetate | 1262 | 1265 | om | - | 0.4 ± 0.03 | 0.8 ± 0.01 |
| isopiperitenone | 1271 | 1272 * | om | 0.2 ± 0.03 | 0.1 ± 0.02 | - |
| trans-sabinyl acetate | 1294 | 1291 | om | - | 3.1 ± 0.41 | 2.0 ± 0.09 |
| β-caryophyllene | 1419 | 1419 | sh | - | - | 0.3 ± 0.02 |
| germacrene D | 1481 | 1485 | sh | 0.5 ± 0.27 | 0.8 ± 0.15 | - |
| β-selinene | 1486 | 1490 | sh | - | 1.0 ± 0.07 | 1.7 ± 0.14 |
| phenylethyl 3-methylbutyrate | 1491 | 1490 | nt | 0.3 ± 0.20 | - | - |
| α-cadinol | 1654 | 1654 | os | - | 0.1 ± 0.02 | - |
| brevifolin | 1669 | 1675 | pp | 23.6 ± 1.21 | 27.9 ± 2.21 | 28.8 ± 1.07 |
| mustakone | 1687 | 1676 | os | - | 0.3 ± 0.01 | - |
| Eudesma-4(15),7-dien-1β -ol | 1688 | 1688 | os | - | 0.3 ± 0.04 | - |
| β-nootkatol | 1712 | 1712 | os | - | 0.3 ± 0.01 | - |
| (Z)-lanceol | 1762 | 1761 | os | - | 1.0 ± 0.14 | - |
| methyl isocostate | 1792 | 1791 | os | - | 0.7 ± 0.21 | - |
| kaurene | 2048 | 2043 | dh | - | 0.2 ± 0.01 | - |
| Chemical Classes | Leaves | Young Inflorescences (Blossom) | Ripe Inflorescences | |||
| Monoterpene hydrocarbons (mh) | 0.9 ± 0.22 B | 1.7 ± 0.03 A | 1.8 ± 0.05 A | |||
| Oxygenated monoterpenes (om) | 74.2 ± 2.01 A | 65.4 ± 2.74 B | 64.7 ± 1.02 B | |||
| Sesquiterpene hydrocarbons (sh) | 0.5 ± 0.27 B | 1.7 ± 0.22 A | 2.0 ± 0.16 A | |||
| Oxygenated sesquiterpenes (os) | - | 2.7 ± 1.59 | - | |||
| Phenylpropanoids (pp) | 23.6 ± 1.21 B | 27.9 ± 2.21 A | 28.8 ± 1.07 A | |||
| Other non-terpene derivatives (nt) | 0.3 ± 0.20 | 0.1 ± 0.03 | - | |||
| Total identified (%) | 99.5 ± 0.12 | 99.7 ± 0.00 | 97.3 ± 0.15 | |||
| OE hydrodistillation yield (% w/w) | 0.35 ± 0.01 B | 0.17 ± 0.01 C | 0.41 ± 0.01 A | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieracci, Y.; Vento, M.; Pistelli, L.; Lombardi, T.; Pistelli, L. Halophyte Artemisia caerulescens L.: Metabolites from In Vitro Shoots and Wild Plants. Plants 2022, 11, 1081. https://doi.org/10.3390/plants11081081
Pieracci Y, Vento M, Pistelli L, Lombardi T, Pistelli L. Halophyte Artemisia caerulescens L.: Metabolites from In Vitro Shoots and Wild Plants. Plants. 2022; 11(8):1081. https://doi.org/10.3390/plants11081081
Chicago/Turabian StylePieracci, Ylenia, Martina Vento, Luisa Pistelli, Tiziana Lombardi, and Laura Pistelli. 2022. "Halophyte Artemisia caerulescens L.: Metabolites from In Vitro Shoots and Wild Plants" Plants 11, no. 8: 1081. https://doi.org/10.3390/plants11081081