Chemical Composition and In Vitro Anti-Helicobacter pylori Activity of Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae) Essential Oil
Abstract
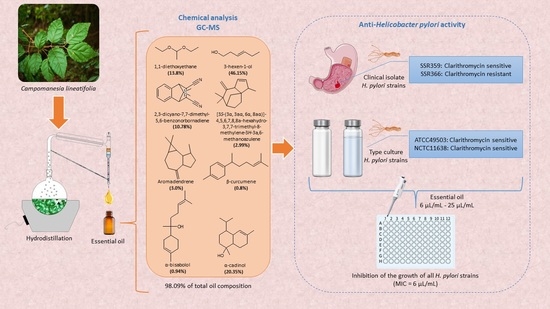
:1. Introduction
2. Results
2.1. Chemical Analysis of the Essential Oil
2.2. In Vitro Anti-Helicobacter pylori Activity Evaluation
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Isolation of the Essential Oil
4.3. Analysis of the Essential Oil
4.4. Anti-Helicobacter pylori Activity
4.4.1. Bacterial Strains and Culture Conditions
4.4.2. Broth Microdilution Assays
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, B.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic and peptic ulceration. Lancet 1984, 323, 1311–1315. [Google Scholar] [CrossRef]
- Smith, S.M. An update on the treatment of Helicobacter pylori infection. EMJ Gastroenterol. 2015, 4, 101–107. [Google Scholar]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H.; et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, e12475. [Google Scholar] [CrossRef]
- Coelho, L.G.V.; Marinho, J.R.; Genta, R.; Ribeiro, L.T.; Passos, M.D.C.F.; Zaterka, S.; Assumpção, P.P.; Barbosa, A.J.A.; Barbuti, R.; Braga, L.L.; et al. IVth Brazilian Consensus Conference on Helicobacter pylori infection. Arq. Gastroenterol. 2018, 16, 97–121. [Google Scholar] [CrossRef]
- Ustün, O.; Ozçelik, B.; Akyön, Y.; Abbasoglu, U.; Yesilada, E. Flavonoids with anti-Helicobacter pylori activity from Cistus laurifolius leaves. J. Ethnopharmacol. 2006, 108, 457–461. [Google Scholar] [CrossRef]
- Bi, W.P. Efficacy and safety of herbal medicines in treating gastric ulcer: A review. World J. Gastroenterol. 2014, 20, 17020–17028. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and antimicrobial-enhancing activity of Chelidonium majus and Corydalis cheilanthifolia extracts against multidrug-resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová, K.; Majerová, M.; Pokorná, M.; Švajdlenka, E. In vitro synergistic effect of Hibiscus sabdariffa aqueous extract in combination with standard antibiotics against Helicobacter pylori clinical isolates. Pharm. Biol. 2016, 54, 1736–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadi, A.T.B. Resistance to clarithromycin and gastroenterologist’s persistence roles in nomination for Helicobacter pylori as high priority pathogen by World Health Organization. World J. Gastroenterol. 2017, 23, 6379–6384. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.G.; O’Brien, M.M.; Gadek, P.A.; Quinn, C.J. Myrtaceae revisited: A reassessment of infrafamilial groups. Am. J. Bot. 2001, 88, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae. In Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. Available online: http://floradobrasil2015.jbrj.gov.br/FB171 (accessed on 8 June 2022).
- Villachica, H.; Carvalho, J.F.U.; Muller, C.H.; Diaz, C.S.; Almanza, M. Frutales y Hortializas Promisorios de la Amazonia. Tratado de Cooperacion Amazonica; Secretaria Pro-Tempore Publicaciones: Lima, Peru, 1996; Volume 44, pp. 212–213. [Google Scholar]
- Lorenzi, H.; Bacher, L.; Lacerda, M.; Sartori, S. Frutas Brasileiras e Exóticas Cultivadas: (de Consumo in Natura), 1st ed.; Instituto Plantarum: São Paulo, Brazil, 2006. [Google Scholar]
- Pio Corrêa, M. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas; Imprensa Nacional: Rio de Janeiro, Brazil, 1952.
- D’Ávilla, M.C. Da Flora Medicinal do Rio Grande do Sul; Faculdade Livre de Medicina e Pharmacia de Porto Alegre: Porto Alegre, Brazil, 1910; p. 155. [Google Scholar]
- Moraes, M. Botânica Brasileira: Applicada à Medicina às Artes e à Indústria (Seguida de um Suplemento de Matéria Médica, Inclusive as Plantas Conhecidas e Applicadas Pelos Índios em Suas Enfermidades); Garnier: Rio de Janeiro, Brazil, 1881. [Google Scholar]
- Lescano, C.H.; Freitas de Lima, F.; Caires, A.R.L.; de Oliveira, I.P. Polyphenols present in Campomanesia Genus: Pharmacological and nutraceutical approach. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 25; pp. 407–420. [Google Scholar]
- Dos Santos, A.L.; Polidoro, A.D.S.; Cardoso, C.A.L.; Batistote, M.; do Carmo Vieira, M.; Jacques, R.A.; Caramão, E.B. GC×GC/qMS analyses of Campomanesia guazumifolia (Cambess.) O. Berg essential oils and their antioxidant and antimicrobial activity. Nat. Prod. Res. 2019, 16, 593–597. [Google Scholar] [CrossRef]
- De Jesus, G.S.; Micheletti, A.C.; Padilha, R.G.; de Souza de Paula, J.; Alves, F.M.; Leal, C.R.B.; Garcez, F.R.; Garcez, W.S.; Yoshida, N.C. Antimicrobial potential of essential oils from cerrado plants against multidrug–resistant foodborne microorganisms. Molecules 2020, 25, 3296. [Google Scholar] [CrossRef]
- Pacheco, L.A.; Ethur, E.M.; Sheibel, T.; Buhl, B.; Weber, A.C.; Kauffmann, C.; Marchi, M.I.; Freitas, E.M.; Hoehne, L. Chemical characterization and antimicrobial activity of Campomanesia aurea against three strains of Listeria monocytogenes. Braz. J. Biol. 2021, 81, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The in vitro activity of essential oils against Helicobacter pylori growth and urease activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.A.; Sallem, O.W.; Abdelhassib, M.R.; Eldahshan, O.A. Potentiation of anti-Helicobacter pylori activity of clarithromycin by Pelargonium graveolens oil. Arab J. Gastroenterol. 2021, 22, 224–228. [Google Scholar] [CrossRef]
- Spósito, L.; Oda, F.B.; Vieira, J.H.; Carvalho, F.A.; dos Santos Ramos, M.A.; de Castro, R.C.; Crevelin, E.J.; Crotti, A.E.M.; Santos, A.G.; da Silva, P.B.; et al. In vitro and in vivo anti-Helicobacter pylori activity of Casearia sylvestris leaf derivatives. J. Ethnopharmacol. 2019, 233, 1–12. [Google Scholar] [CrossRef]
- Madalosso, R.C.; Oliveira, G.C.; Martins, M.T.; Vieira, A.E.D.; Barbosa, J.; Caliari, M.V.; Castilho, R.O.; Tagliati, C.A. Campomanesia lineatifolia Ruiz & Pav. as a gastroprotective agent. J. Ethnopharmacol. 2012, 139, 772–779. [Google Scholar]
- Henriques, B.O.; Corrêa, O.; Azevedo, E.P.C.; Pádua, R.M.; Oliveira, V.L.S.D.; Oliveira, T.H.C.; Boff, D.; Dias, A.C.F.; Souza, D.G.D.; Amaral, F.A.; et al. In vitro TNF-α inhibitory activity of brazilian plants and anti-inflammatory effect of Stryphnodendron adstringens in an acute arthritis model. Evid. Based Complement. Altern. Med. 2016, 2016, 9872598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willner, B.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in bartlett pear brandies by means of the sensomics concept. J. Agric. Food Chem. 2013, 61, 9583–9593. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, B.; Porcedda, S.; Porta, G.D.; Reverchon, E. Extraction and isolation of Salvia desoleana and Mentha spicata subsp. insularis essential oils by supercritical CO2. Flavour Fragr. J. 2001, 16, 384–388. [Google Scholar] [CrossRef]
- Kang, S.H.; Monti, S.A. Revised structure of zizanol. J. Org. Chem. 1984, 49, 3830–3832. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; Kazemipoor, N.; Valizadeh, J.; Falak Nezhad Seifi, M.; Rahneshan, N. Essential oil composition of Eucalyptus microtheca and Eucalyptus viminalis. Avicenna J. Phytomed. 2015, 5, 540–552. [Google Scholar]
- Lucero, M.E.; Estell, R.E.; Fredrickson, E.L. The essential oil composition of Psorothamnus scoparius (A. Gray) RybB. J. Essent. Oil Res. 2003, 15, 108–111. [Google Scholar] [CrossRef]
- Zheng, C.H.; Kim, T.H.; Kim, K.H.; Leem, Y.H.; Lee, H.J. Characterization of potent aroma compounds in Chrysanthemum coronarium L. (Garland) using aroma extract dilution analysis. Flavour Fragr. J. 2004, 19, 401–405. [Google Scholar] [CrossRef]
- Yu, E.J.; Kim, T.H.; Kim, K.H.; Lee, H.J. Aroma-active compounds of Pinus densiflora (red pine) needles. Flavour Fragr. J. 2004, 19, 532–537. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Osorio, C.; Alarcon, M.; Moreno, C.; Bonilla, A.; Barrios, J.; Garzon, C.; Duque, C. Characterization of odor-active volatiles in champa (Campomanesia lineatifolia R. & P.). J. Agric. Food Chem. 2006, 54, 509–516. [Google Scholar]
- Craveiro, A.A.; Fernandes, G.F.; Andrade, C.H.S. Óleos Essenciais de Plantas do Nordeste; Edições UFC: Fortaleza, Brazil, 1981. [Google Scholar]
- Abdelgaleil, S.A.M. Chemical composition, insecticidal and fungicidal activities of essential oils isolated from Mentha microphylla and Lantana camara growing in Egypt. Alex. Sci. Exch. J. 2006, 27, 18–28. [Google Scholar]
- Coffie, E. Essential Oils and Compounds Isolated from the Leaves and Rhizomes of Aframomum atewae. Ph.D. Thesis, University of Ghana, Accra, Ghana, July 2019. [Google Scholar]
- Martinez, J.; Rosa, P.T.V.; Menut, C.; Leydet, A.; Brat, P.; Pallet, D.; Meireles, M.A.A. Valorization of brazilian vetiver (Vetiveria zizanioides (L.) nash ex small) oil. J. Agric. Food Chem. 2004, 52, 6578–6584. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Kumar, R.; Kaushik, S.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P.S. Antioxidant potential of the root of Vetiveria zizanioides (L.) nash. Indian J. Biochem. Biophys. 2009, 46, 122–125. [Google Scholar] [PubMed]
- Luqman, S.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P.S. Detection of antibacterial activity in spent roots of two genotypes of aromatic grass Vetiveria zizanioides. Pharm. Biol. 2005, 43, 732–736. [Google Scholar] [CrossRef]
- Balasankar, D.; Vanilarasu, K.; Selva Preetha, P.; Rajeswari, M.; Umadevi, S.; Bhowmik, D. Traditional and medicinal uses of vetiver. J. Med. Plants Stud. 2013, 1, 191–200. [Google Scholar]
- Yangui, I.; Zouaoui Boutiti, M.; Boussaid, M.; Messaoud, C. Essential oils of myrtaceae species growing wild in tunisia: Chemical variability and antifungal activity against Biscogniauxia mediterranea, the causative agent of charcoal canker. Chem. Biodivers. 2017, 14, e1700058. [Google Scholar] [CrossRef]
- García-Díaz, E.; Trejo, R.; Tafoya, F.; Aragón-García, A.; Elizalde-González, M.P. Profile of terpenoid compounds mediating a plant-herbivore interaction: Screening by static headspace solid-phase microextraction-gas chromatography/Q-ToF mass spectrometry. Chem. Biodivers. 2020, 17, e2000564. [Google Scholar] [CrossRef]
- Woollard, J.M.R.; Perry, N.B.; Weavers, R.T.; van Klink, J.W. Bullatenone, 1,3-dione and sesquiterpene chemotypes of Lophomyrtus species. Phytochemistry 2008, 69, 1313–1318. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Srivastava, S.K.; Shah, N.C. Constituents of the rhizome essential oil of Curcuma amada Roxb. from India. J. Essent. Oil Res. 2001, 13, 63–64. [Google Scholar] [CrossRef]
- Ministério da Saúde. Monografia da Espécie Curcuma longa L. (curcuma); Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2015.
- Jain, S.K. Ethnobotanical diversity in zingibers of India. Ethnobotany 1995, 7, 83–88. [Google Scholar]
- Zaidi, S.F.H.; Yamada, K.; Kadowaki, M.; Usmanghani, K.; Sugiyama, T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009, 121, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Koosirirat, C.; Linpisarn, S.; Changsom, D.; Chawansuntati, K.; Wipasa, J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int. Immunopharmacol. 2010, 10, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Sarrazin, S.; Oliveira, R.; Suemitsu, C.; Maia, J.; Mourão, R. Composition and antimicrobial activity of leaf essential oils of Myrcia sylvatica (G. Mey.) DC. Eur. J. Med. Plants 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Vila, R.; Santana, A.I.; Pérez-Rosés, R.; Valderrama, A.; Castelli, M.V.; Mendonca, S.; Zacchino, S.; Gupta, M.P.; Cañigueral, S. Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of α-bisabolol. Bioresour. Technol. 2010, 101, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; Batista, H.R.F.; Estevam, E.B.B.; Alves, C.C.F.; Forim, M.R.; Nicolella, H.D.; Furtado, R.A.; Tavares, D.C.; Silva, T.S.; Martins, C.H.G.; et al. Chemical composition and in vitro antibacterial and antiproliferative activities of the essential oil from the leaves of Psidium myrtoides O. Berg (Myrtaceae). Nat. Prod. Res. 2019, 33, 2566–2570. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, K.G.; Kang, J.Y.; Yap, I.; Guan, R.; Tan, C.C.; Wee, A.; Teng, C.H. Chili protects against aspirin-induced gastroduodenal mucosal injury in humans. Dig. Dis. Sci. 1995, 40, 580–583. [Google Scholar] [CrossRef]
- Bortolotti, M.; Coccia, G.; Grossi, G.; Miglioli, M. The treatment of functional dyspepsia with red pepper. Aliment. Pharmacol. Ther. 2002, 16, 1075–1082. [Google Scholar] [CrossRef]
- Yongwatana, K.; Harinwan, K.; Chirapongsathorn, S.; Opuchar, K.; Sanpajit, T.; Piyanirun, W.; Puttapitakpong, C. Curcuma longa Linn versus omeprazole in treatment of functional dyspepsia: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2022, 37, 335–341. [Google Scholar] [CrossRef]
- Santos-Oliveira, R.; Coulaud-Cunha, S.; Colaço, W. Revisão da Maytenus ilicifolia Mart. ex Reissek, Celastraceae. Contribuição ao estudo das propriedades farmacológicas. Rev. Bras. Farmacogn. 2009, 19, 650–659. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Tang, X.; Peng, L.; Li, X.; Zhao, G.; Zhong, L. Chemical composition, antimicrobial and antioxidant activities of the flower volatile oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum Cymosum. Molecules 2018, 23, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pino, J.A.; Gaviria, M.; Quevedo-Vega, J.; García-Lesmes, L.; Quijano-Celis, C.E. Essential oil of Galinsoga parviflora leaves from Colombia. Nat. Prod. Commun. 2010, 5, 1831–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, A.; Wang, F.; Sun, X.; Li, H.; Lin, J.; Li, P.; Deng, G. Chemical composition, antioxidant, and antimicrobial activities of Vetiveria zizanioides (L.) nash essential oil extracted by carbon dioxide expanded ethanol. Molecules 2019, 24, 1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Cardoso, C.A.L.; Salmazzo, G.R.; Honda, N.K.; Prates, C.B.; Vieira, M.D.C.; Coelho, R.G. Antimicrobial activity of the extracts and fractions of hexanic fruits of Campomanesia species (Myrtaceae). J. Med. Food. 2010, 13, 1273–1276. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [Green Version]
- Bergonzelli, G.E.; Donnicola, D.; Porta, N.; Corthésy-Theulaz, I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003, 47, 3240–3246. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Kita, M.; Yamaoka, Y.; Imamura, S.; Yamamoto, T.; Mitsufuji, S.; Kodama, T.; Kashima, K.; Imanishi, J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter 2003, 8, 207–215. [Google Scholar] [CrossRef]
- Patel, J.B.; Cockerill, F.R.; Bradford, P.A. M100-S25 performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. Clin. Lab. Stand. Inst. 2015, 35, 29–50. [Google Scholar]
- Borges, A.S.; Minozzo, B.R.; Santos, H.; Ardisson, J.S.; Rodrigues, R.P.; Romão, W.; de Souza Borges, W.; Gonçalves, R.D.C.R.; Beltrame, F.L.; Kitagawa, R.R. Plectranthus barbatus Andrews as anti-Helicobacter pylori agent with activity against adenocarcinoma gastric cells. Ind. Crops Prod. 2020, 146, 112207. [Google Scholar] [CrossRef]

| Compound | RT (min) | RI | Molecular Weight | Molecular Formula | % | MS | ID |
|---|---|---|---|---|---|---|---|
| 1,1-diethoxyethane (1) | 2.483 | 718 | 118 | C6H14O2 | 13.08 | 103, 75, 73, 61, 47, 45, 43, 31, 29 | NIST; Willner, Granvogl & Schieberle (2013) [29] |
| 3-hexen-1-ol (2) | 3.523 | 850 | 100 | C6H12O | 46.15 | 82, 79, 72, 69, 67, 65, 57, 55, 53, 51, 41, 39 | NIST; Marongiu et al. (2001) [30] |
| 2,3-dicyano-7,7-dimethyl-5,6-benzonorbornadiene (3) | 18.158 | 1506 | 220 | C15H12N2 | 10.78 | 223, 221, 207, 206, 205 | Wiley |
| [3-S-(3α, 3aα, 6α, 8aα)]-4,5,6,7,8,8a-hexahydro-3,7,7-trimethyl-8-methylene-3H-3a,6-methanoazulene (4) | 20.044 | 1586 | 202 | C15H22 | 2.99 | 187, 159, 145, 131, 119, 105, 91, 77 | Kang & Monti (1984) [31] |
| Aromadendrene 2 (5) | 20.275 | 1596 | 204 | C15H24 | 3.0 | 161, 133, 119, 105, 93, 91, 81, 79 | NIST; Maghsoodlou et al. (2015) [32] |
| β-curcumene (6) | 21.547 | 1652 | 204 | C15H24 | 0.8 | 161, 119, 105, 93, 81, 55, 41 | Wiley; Lucero, Estell & Fredrickson (2003) [33] |
| α-bisabolol (7) | 21.555 | 1652 | 222 | C15H26O | 0.94 | 204, 161, 119, 105, 93, 69, 41 | NIST; Zheng et al. (2004) [34] |
| α-cadinol (8) | 21.809 | 1664 | 222 | C15H26O | 20.35 | 204, 189, 161, 134, 121, 109, 105, 95, 81, 71 | NIST; Yu et al. (2004) [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, N.C.V.; de Mello, M.P.; Smith, S.M.; Boylan, F.; Caliari, M.V.; Castilho, R.O. Chemical Composition and In Vitro Anti-Helicobacter pylori Activity of Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae) Essential Oil. Plants 2022, 11, 1945. https://doi.org/10.3390/plants11151945
Neves NCV, de Mello MP, Smith SM, Boylan F, Caliari MV, Castilho RO. Chemical Composition and In Vitro Anti-Helicobacter pylori Activity of Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae) Essential Oil. Plants. 2022; 11(15):1945. https://doi.org/10.3390/plants11151945
Chicago/Turabian StyleNeves, Nívea Cristina Vieira, Morgana Pinheiro de Mello, Sinéad Marian Smith, Fabio Boylan, Marcelo Vidigal Caliari, and Rachel Oliveira Castilho. 2022. "Chemical Composition and In Vitro Anti-Helicobacter pylori Activity of Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae) Essential Oil" Plants 11, no. 15: 1945. https://doi.org/10.3390/plants11151945