THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying Diversity to Underpin Future Cannabis Breeding
Abstract
:1. Introduction
2. Results
2.1. THC and CBD Quantification and Phenotyping
2.2. Correlation between the Climatic and Geographical Parameters and THC and CBD Quantity Variation
3. Discussion
3.1. THC and CBD Diversity
3.2. Morphological Features and Phenotyping
3.3. Phenotyping in Terms of THC and CBD Composition
3.4. Effect of Climatic and Geographical Characteristics on THC and CBD Content Variation
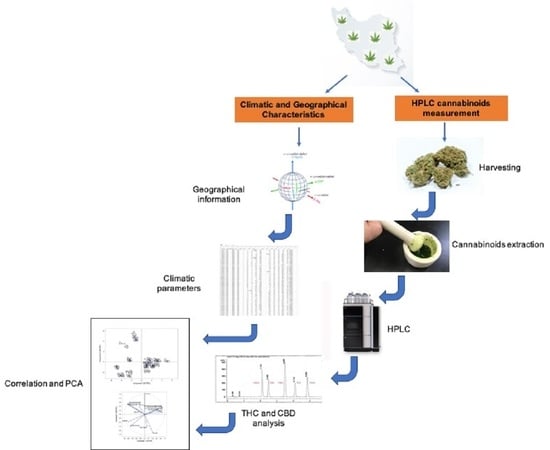
4. Materials and Methods
4.1. Genetic Resources
4.2. Sample Preparation
4.3. Chemicals
4.4. THC and CBD Standard Preparation
4.5. HPLC analysis of Cannabinoids
4.6. Climatic and Geographical Data
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet. Resour. Crop Evol. 2005, 52, 161–180. [Google Scholar] [CrossRef]
- Callaway, J.C.; Pate, W.D. Hempseed oil. AOCS 2009, 21, 185–213. [Google Scholar]
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B 2009, 877, 4115–4124. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.; Linthorst, H.J.M.; Verpoorte, R. In silico expression analysis of PKS genes isolated from Cannabis sativa L. Genet. Mol. Biol. 2010, 33, 703–713. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, S.; Fischedick, J.; Podkolinski, R.; Raber, J.C. Cannabinoids and Terpenes as Chemotaxonomic Markers in Cannabis. Nat. Prod. Chem. Res. 2015, 3, 9. [Google Scholar]
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent advances in Cannabis sativa genomics research. New Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Raharjo, J.T.; Widjaja, I.; Roytrakul, S.; Verpoorte, R. Comparative Proteomics of Cannabis sativa Plant Tissues. J. Biomol. Tech. 2004, 15, 97–106. [Google Scholar]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The Gene Controlling Marijuana Psychoactivity. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Marks, D.M.; Tian, L.; Wenger, J.P.; Omburo, S.N.; Soto-Fuentes, W.; He, J.; Gang, D.R.; Weiblen, G.D.; Dixon, R.A. Identification of candidate genes affecting D9-tetrahydrocannabinol biosynthesis in Cannabis sativa. J. Exp. Bot. 2009, 60, 3715–3726. [Google Scholar] [CrossRef] [Green Version]
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, S.J.; Quilichini, T.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2019, 101, 37–56. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, D.; Miselli, F.; Carboni, A. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- Potter, D.J.; Clark, P.; Brown, M.B. Potency of ∆9–THC and other cannabinoids in cannabis in England in 2005: Implications for psychoactivity and pharmacology. J. Forensic Sci. 2008, 53, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.B. Cannabidiol claims and misconceptions. Trends Pharm. Sci. 2017, 38, 198–201. [Google Scholar] [CrossRef]
- Small, E.; Marcus, D. A New Crop with New Uses for North America. ASHS 2002, 24, 284–326. [Google Scholar]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zhang, X.; Li, Y.; Ridout, K.; Serrano-Serrano, M.L.; Yang, Y.; Liu, A.; Ravikanth, G.; Nawaz, M.A.; Mumtaz, A.S.; et al. Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci. Adv. 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Rustichelli, C.; Ferioli, V.; Baraldi, M.; Zanoli, P.; Gamberini, G. Analysis of cannabinoids in fiber hemp plant varieties (Cannabis sativa L.) by high-performance liquid chromatography. Chromatographia 1998, 47, 215–222. [Google Scholar] [CrossRef]
- Mandolino, G.; Bagatta, M.; Carboni, A.; Ranalli, P.; De Meijer, E. Qualitative and quantitative aspects of the inheritance of chemical phenotype in Cannabis. J. Ind. Hemp 2003, 8, 51–57. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovano, A.; Bachari, A.; Arad, N.; Mantri, N. It is our turn to get cannabis high: Put cannabinoids in food and health baskets. Molecules 2020, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Stuart, J.D.; Li, Y.; Parnas, R.S. The feasibility of converting Cannabis sativa L. oil into biodiesel. Bioresour. Technol. 2010, 101, 8457–8460. [Google Scholar] [CrossRef]
- Jikomes, N.; Zoorob, M. The cannabinoid content of legal Cannabis in Washington state varies systematically across testing facilities and popular consumer products. Sci. Rep. 2018, 8, 1–15. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Hillig, K.W. A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochem. Syst. Ecol. 2004, 32, 875–891. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Micheler, M.; Carboni, A.; Ranalli, P.; Mandolino, G. Genetics and marker-assisted selection of the chemotype in Cannabis sativa L. Mol. Breed. 2006, 17, 257–268. [Google Scholar] [CrossRef]
- Kojoma, M.; Seki, H.; Yoshida, S.; Muranaka, T. DNA polymorphism in the tetrahydrocannabinolic acid (THCA) synthase gene in ‘‘drug-type’’ and ‘‘fiber-type’’ Cannabis sativa L. Forensic Sci. Int. 2006, 159, 132–140. [Google Scholar] [CrossRef]
- Piluzza, G.; Delogu, G.; Cabras, A.; Marceddu, S.; Bullitta, S. Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet. Resour. Crop Evol. 2013, 60, 2331–2342. [Google Scholar] [CrossRef]
- Grassa, C.J.; Wenger, J.P.; Dabney, C.; Poplawski, S.G.; Motley, S.T.; Michael, T.P.; Schwartz, C.J.; Weiblen, G.D. A complete Cannabis chromosome assembly and adaptive admixture for elevated cannabidiol (CBD) content. New Phytol. 2018, 230, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Pichini, S.; Mannocchi, G.; Berretta, P.; Zaami, S.; Pirani, F.; Pacifici, R.; Busardo, F.P. Δ9-Tetrahydrocannabinol and cnnabidiol time courses in the Sera of “light cannabis” smokers: Discriminating light cannabis use from illegal and medical cannabis use. Ther. Drug Monit. 2020, 42, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Crippa, J.A.S.; Hallak, J.E.C.; Moreira, F.A.; Guimaraes, F.S. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med. Biol. Res. 2006, 39, 421–429. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachari, A.; Piva, T.J.; Salami, S.A.; Jamshidi, N.; Mantri, N. Roles of cannabinoids in melanoma: Evidence from in vivo studies. Int. J. Mol. Sci. 2020, 21, 6040. [Google Scholar] [CrossRef] [PubMed]
- Kojoma, M.; Iida, O.; Makino, Y.; Sekita, S.; Satake, M. DNA fingerprinting of Cannabis sativa using Inter-Simple Sequence Pepeat (ISSR) amplification. Planta Med. 2001, 68, 60–63. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; van der Kamp, H.J.; van Eeuwijk, F.A. Characterisation of Cannabis accessions with regard to cannabinoid content in relation to other plant characters. Euphytica 1992, 62, 187–200. [Google Scholar] [CrossRef]
- Marchei, E.; Tittarelli, R.; Pellegrini, M.; Rotolo, M.C.; Pacifici, R.; Pichini, S. Is “light cannabis” really light? Determination of cannabinoids content in commercial products. Clin. Chem. Lab. Med. 2020, 58, 175–177. [Google Scholar] [CrossRef]
- Anwar, F.; Latifa, S.; Ashraf, M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. J. Am. Oil Chem. Soc. 2006, 83, 323–329. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects and analytical determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, G.; Carroll, E.L.; Bruford, M.W.; DeWoody, J.A.; Strand, A.; Waits, L.; Wang, J. Next-generation metrics for monitoring genetic erosion within populations of conservation concern. Evol. Appl. 2018, 11, 1066–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danziger, N.; Bernstein, N. Shape Matters: Plant architecture affects chemical uniformity in large-size medical Cannabis plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of phenotypic characteristics in three chemotype categories in the genus Cannabis. HortScience 2021, 56, 481–490. [Google Scholar] [CrossRef]
- Weiblen, G.D.; Wenger, J.P.; Craft, K.J.; ElSohly, M.A.; Mehmedic, Z.; Treiber, E.L.; Marks, M.D. Gene duplication and divergence affecting drug content in Cannabis sativa. New Phytol. 2015, 208, 1241–1250. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in Cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Mechtler, K.; Bailer, J.; de Hueber, K. Variations of ∆9-THC content in single plants of hemp varieties. Ind. Crop. Prod. 2004, 19, 19–24. [Google Scholar] [CrossRef]
- Onofri, C.; de Meijer, E.P.M.; Mandolino, G. Sequence heterogeneity of cannabidiolic- and tetrahydrocannabinolic acid-synthase in Cannabis sativa L. and its relationship with chemical phenotype. Phytochemistry 2015, 116, 57–68. [Google Scholar] [CrossRef]
- Lynch, R.C.; Vergara, D.; Tittes, S.; White, K.; Schwartz, C.J.; Gibbs, M.J.; Ruthenburg, T.C.; Decesare, K.; Land, D.P.; Kane, N.C. Genomic and Chemical Diversity in Cannabis. Crit. Rev. Plant Sci. 2016, 35, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Zager, J.; Lange, I.; Srividya, N.; Smith, A.; Lange, B.M. Gene networks underlying cannabinoid and terpenoid accumulation in cannabis. Plant Physiol. 2019, 180, 1877–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blebea, N.M.; Rambu, D.; Costache, T.; Negres, S. Very fast RP–UHPLC–PDA method for identification and quantification of the cannabinoids from hemp oil. Appl. Sci. 2021, 11, 9414. [Google Scholar] [CrossRef]
- Mandrioli, M.; Tura, M.; Scotti, S.; Toschi, T.G. Fast Detection of 10 cannabinoids by RP-HPLC-UV method in Cannabis sativa L. Molecules 2019, 24, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madej, K.; Chmiołek, A.; Szlachta, K.; Piekoszewski, W. HPLC-DAD analysis of hemp oil supplements for determination of four cannabinoids: Cannabidiol, cannabidiolic acid, cannabinol and delta 9-Tetrahydrocannabinol. Separations 2021, 8, 227. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Wenger, J.P.; Dabney, C.J., III; ElSohly, M.A.; Chandra, S.; Radwan, M.M.; Majumdar, C.G.; Weiblen, G.D. Validating a predictive model of cannabinoid inheritance with feral, clinical, and industrial Cannabis sativa. Am. J. Bot. 2020, 107, 1423–1432. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of chemotypic markers in three chemotype categories of Cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark, and roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Sawler, J.; Stout, J.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The genetic structure of marijuana and hemp. PLoS ONE 2015, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Soorni, A.; Fatahi, R.; Haak, D.C.; Salami, S.A.; Bombarely, A. Assessment of genetic diversity and population structure in Iranian cannabis germplasm. Sci. Rep. 2017, 7, 10. [Google Scholar]
- Bennici, A.; Mannucci, C.; Calapai, F.; Cardia, L.; Ammendolia, I.; Gangemi, S.; Calapai, G.; Soler, D.G. Safety of medical Cannabis in neuropathic chronic pain management. Molecules 2021, 26, 6257. [Google Scholar] [CrossRef]
- Hilliard, A.; Stott, C.; Wright, S.; Guy, G.; Pryce, G.; Al-Izki, S.; Bolton, C.; Giovannoni, G. Evaluation of the Effects of Sativex (THC BDS: CBD BDS) on Inhibition of Spasticity in a Chronic Relapsing Experimental Allergic Autoimmune Encephalomyelitis: A Model of Multiple Sclerosis. ISRN Neurol. 2012, 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. Ist. Super Sanita 2020, 56, 285–291. [Google Scholar]
- Zuardi, A.W. Cannabidiol: From an inactive cannabinoid to a drug with wide spectrum of action. Bras. J. Psychiatry 2008, 30, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Zuardi, A.W.; Crippa, J.A.S.; Hallak, J.E.C.; Pinto, J.P.; Chagas, M.H.N.; Rodrigues, G.G.R.; Dursun, S.M.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooshangi Shayesteh, M.R.; Haghi-Aminjan, H.; Baeeri, M.; Rahimifard, M.; Hassani, S.; Gholami, M. Modification of the hemodynamic and molecular features of phosphine, a potent mitochondrial toxicant in the heart, by cannabidiol. Toxicol. Mech. Meth. 2021, 85, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.C.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders. Front. Pharmacol. 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018, 56, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C. Traditional Cannabis cultivation in Darchula District, Nepal- seed, resin and textiles. J. Ind. Hemp 2007, 12, 19–42. [Google Scholar] [CrossRef]
- Mehrani, S.; Azarnivand, H.; Pourtahmasi, K.; Salami, S.A. Comprehensive evaluation of native hemp populations using wood anatomy and stem fiber biometry characteristics towards producing fibers. For. Wood Prod. 2019, 72, 135–145. [Google Scholar]
- Corroon, J.; Kight, R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018, 3, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Phillips, J.A. A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res. 2018, 3, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, A.; del Río, J.C. Chemical characterization of pitch deposits produced in the manufacturing of high-quality paper pulps from hemp fibers. Bioresour. Technol. 2005, 96, 1445–1450. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, G.; Hausman, J.F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar]
- Pate, D.W. Possible role of ultraviolet radiation in evolution of Cannabis chemotypes. Econ. Bot. 1983, 37, 396–405. [Google Scholar] [CrossRef]
- Bruci, Z.; Papoutsis, I.; Athanaselis, S.; Nikolaou, P.; Pazari, E.; Spiliopoulou, C.; Vyshka, G. First systematic evaluation of the potency of Cannabis sativa plants grown in Albania. Forensic Sci. Int. 2012, 222, 40–46. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Mehmedic, Z.; Khan, I.A.; ElSohly, M.A. Assessment of cannabinoids content in micropropagated plants of Cannabis sativa and their comparison with conventionally propagated plants and mother plant during developmental stages of growth. Planta Med. 2010, 76, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Zoller, O.; Rhyn, P.; Zimmerli, B. High-performance liquid chromatographic determination of delta9-tetrahydrocannabinol and the corresponding acid in hemp containing foods with special regard to the fluorescence properties of delta9-tetrahydrocannabinol. J. Chromatogr. A 2000, 872, 101–110. [Google Scholar] [CrossRef]
- Kondratovitch, L.; Scotti, A. Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products; UNODC: Vienna, Austria, 2009; pp. 1–50. [Google Scholar]
- Swift, W.; Wong, A.; Li, K.M.; Arnold, J.C.; McGregor, I.S. Analysis of Cannabis Seizures in NSW, Australia: Cannabis Potency and Cannabinoid Profile. PLoS ONE 2013, 8, e70052. [Google Scholar] [CrossRef]
- Patel, B.; Wene, D.; Fan, Z. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Salami, S.A.; Sharifi, M.; Sohrabi, S. Signaling compounds elicit expression of key genes in cannabinoid pathway and related metabolites in cannabis. Ind. Crops Prod. 2019, 133, 105–110. [Google Scholar] [CrossRef]
- Zivovinovic, S.; Alder, R.; Allenspach, M.D.; Steuer, S. Determination of cannabinoids in Cannabis sativa L. samples for recreational, medical, and forensic purposes by reversed-phase liquid chromatography-ultraviolet detection. J. Anal. Sci. Technol. 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Salari, F.; Mansori, H. The effects of jamonate on plastidal terpenoids on Cannabis sativa L. at vegetative stage. Biol. Plant. 2012, 56, 153–156. [Google Scholar]
- Gambaro, V.; Dell’Acqua, L.; Farè, F.; Froldi, R.; Saligari, E.; Tassoni, G. Determination of primary active constituents in Cannabis preparations by high-resolution gas chromatography/flame ionization detection and high-performance liquid chromatography/UV detection. Anal. Chim. Acta 2002, 468, 245–254. [Google Scholar] [CrossRef]







| Variables | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC | CBD | Longitude (E) | Latitude (N) | Elevation (m) | WS 1 | Tmean 2 | Tmin 3 | Tmax 4 | RHmean 5 | RHmin 6 | RHmax 7 | SD 8 | P 9 | DP 10 | |
| THC | 1 | −0.053 NS | 0.118 NS | −0.157 * | −0.408 ** | −0.326 ** | 0.352 ** | 0.292 ** | 0.412 ** | −0.103 NS | −0.206 ** | −0.057 NS | −0.039 NS | −0.114 NS | 0.305 ** |
| CBD | −0.053 NS | 1 | 0.042 NS | −0.239 ** | −0.222 ** | −0.040 NS | 0.314 ** | 0.311 ** | 0.287 ** | −0.201 * | −0.171 * | −0.188 * | 0.150 NS | −0.028 NS | 0.149 NS |
| Country | Province | Location Code | Sample Size | Elevation (m) | Longitude (E) | Latitude (N) | Annual Average Temperature (C) | Annual Rainfall (mm) | Seed to Harvest (Day) |
|---|---|---|---|---|---|---|---|---|---|
| Iran | West Azerbaijan | Pir-01 | 7 | 1572 | 45°08′ | 36°42′ | 13.28 | 640.61 | 50 |
| Iran | Kurdistan | San-01 | 9 | 1464 | 46°99′ | 35°31′ | 14.00 | 375.10 | 209 |
| Iran | Hamadan | Nhv-01 | 8 | 1666 | 48°25′ | 34°15′ | 14.72 | 385.29 | 198 |
| Iran | Kurdistan | Ban-01 | 10 | 1503 | 45°53′ | 35°59′ | 14.26 | 660.88 | 206 |
| Iran | Kurdistan | Sqz-01 | 7 | 1480 | 46°26′ | 36°24′ | 11.20 | 439.37 | 53 |
| Iran | Kerman | Sir-01 | 6 | 1754 | 55°68′ | 29°43′ | 17.81 | 138.52 | 207 |
| Iran | Ardabil | Ard-01 | 6 | 1339 | 48°29′ | 38°24′ | 9.12 | 273.67 | 165 |
| Iran | Sistan and Balochistan | Zah-01 | 10 | 1352 | 60°86′ | 29°49′ | 19.30 | 73.58 | 208 |
| Iran | Khuzestan | Rmhz-01 | 6 | 179 | 49°59′ | 31°27′ | 27.67 | 280.49 | 210 |
| Iran | Markazi | Ark-01 | 9 | 1722 | 49°42′ | 34°04′ | 14.04 | 297.75 | 200 |
| Iran | Qazvin | Qzv-01 | 10 | 1315 | 49°86′ | 36°47′ | 13.91 | 311.85 | 183 |
| Iran | West Azerbaijan | Naq-01 | 7 | 1324 | 45°22′ | 36°57′ | 14.08 | 323.95 | 58 |
| Iran | Zanjan | Abh-01 | 9 | 1543 | 49°02′ | 36°28′ | 12.39 | 301.31 | 179 |
| Iran | Hamadan | Sam-01 | 6 | 1858 | 48°70′ | 34°20′ | 13.53 | 324.79 | 198 |
| Iran | Kerman | Krmn-01 | 7 | 1761 | 56°58′ | 30°15′ | 17.01 | 123.43 | 185 |
| Iran | Kermanshah | Krsh-01 | 7 | 1389 | 47°03′ | 34°19′ | 15.51 | 402.63 | 206 |
| Iran | Kerman | Bam-01 | 10 | 1068 | 58°14′ | 29°09′ | 23.74 | 54.05 | 208 |
| Iran | South Khorasan | Bsh-01 | 10 | 881 | 57°43′ | 34°03′ | 21.07 | 79.98 | 174 |
| Iran | Khuzestan | Dez-01 | 9 | 144 | 48°42′ | 32°38′ | 24.56 | 389.40 | 206 |
| Iran | Khuzestan | Dez-02 | 8 | 144 | 48°42′ | 32°38′ | 24.56 | 389.40 | 201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafaei Dehnavi, M.; Ebadi, A.; Peirovi, A.; Taylor, G.; Salami, S.A. THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying Diversity to Underpin Future Cannabis Breeding. Plants 2022, 11, 129. https://doi.org/10.3390/plants11010129
Mostafaei Dehnavi M, Ebadi A, Peirovi A, Taylor G, Salami SA. THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying Diversity to Underpin Future Cannabis Breeding. Plants. 2022; 11(1):129. https://doi.org/10.3390/plants11010129
Chicago/Turabian StyleMostafaei Dehnavi, Mahboubeh, Ali Ebadi, Afshin Peirovi, Gail Taylor, and Seyed Alireza Salami. 2022. "THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying Diversity to Underpin Future Cannabis Breeding" Plants 11, no. 1: 129. https://doi.org/10.3390/plants11010129