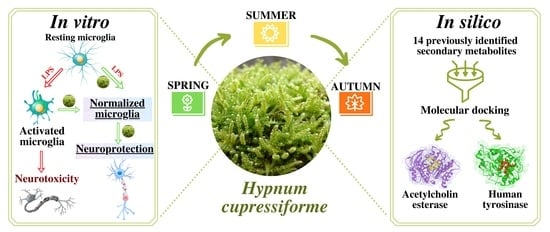
The Influence of Seasonality on Secondary Metabolite Profiles and Neuroprotective Activities of Moss Hypnum cupressiforme Extracts: In Vitro and In Silico Study
Abstract
:1. Introduction
2. Results
2.1. (Bio)Chemical Evaluation
2.2. Anti-Neuroinflammatory Potential of H. cupressiforme Extracts
2.2.1. H2O2-Stimulated BV2 Microglial Cells
2.2.2. LPS-Stimulated BV2 Microglial Cells
2.3. Neuroprotective Potential of H. cupressiforme Extracts
2.4. Anti-Neurodegenerative Potential of H. cupressiforme Extracts
2.5. In Silico Molecular Docking
2.5.1. AchE Docking
2.5.2. Tyrosinase (mTyr and hTyr) Docking
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Selected Classes of Secondary Metabolites
4.3. Antioxidant Activity
4.4. Anti-Neuroinflammatory Activity
4.4.1. Cell Culture
4.4.2. Stimulation of BV2 Microglial Cells
4.4.3. Microglial Culture Supernatant Transfer Model
4.4.4. MTT Assay
4.4.5. NBT Assay
4.4.6. Griess Assay
4.4.7. Measurement of Cytokine Levels in Cell Supernatants
4.5. Anti-Neurodegenerative Activity
4.6. Molecular Docking
Docking Procedure
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extracts (moss phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef] [Green Version]
- Bargagli, R.; Monaci, F.; Borghini, F.; Bravi, F.; Agnorelli, C. Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 2002, 116, 279–287. [Google Scholar] [CrossRef]
- Glime, J. Medical Uses: Medical Conditions. Bryophyt. Ecol. 2017, 5, 1–25. Available online: https://digitalcommons.mtu.edu/cgi/viewcontent.cgi?article=1139&context=bryo-ecol-subchapters (accessed on 1 October 2021).
- Glime, J.M. Medical Uses: Biologically Active Substances. Bryophyt. Ecol. 2017, 5, 517. [Google Scholar]
- Sabovljević, M.S.; Sabovljević, A.D.; Ikram, N.K.K.; Peramuna, A.; Bae, H.; Simonsen, H.T. Bryophytes—An emerging source for herbal remedies and chemical production. Plant Genet. Resour. 2016, 14, 314–327. [Google Scholar] [CrossRef]
- Mandić, M.R.; Oalđe, M.M.; Lunić, T.M.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Božić Nedeljković, B.D. Chemical characterization and in vitro immunomodulatory effects of different extracts of moss Hedwigia ciliata (Hedw.) P. Beauv. from the Vršačke Planine Mts., Serbia. PLoS ONE 2021, 16, e0246810. [Google Scholar] [CrossRef] [PubMed]
- Ros, R.M.; Mazimpaka, V.; Abou-Salama, U.; Aleffi, M.; Blockeel, T.L.; Brugués, M.; Cros, R.M.; Dia, M.G.; Dirkse, G.M.; Draper, I. Mosses of the Mediterranean, an annotated checklist. Cryptogam. Bryol. 2013, 34, 99–283. [Google Scholar] [CrossRef]
- Pantović, J.; Veljić, M.; Grdović, S.; Sabovljević, M.S. An annotated list of moss species of Serbia. Phytotaxa 2021, 479, 207–249. [Google Scholar] [CrossRef]
- Yayıntaş, O.T.; Yılmaz, S.; Sökmen, M. Determination of antioxidant, antimicrobial and antitumor activity of bryophytes from Mount Ida (Canakkale, Turkey). Indian J. Tradit. Knowl. 2019, 18(2), 395–401. [Google Scholar]
- Lunić, T.M.; Oalđe, M.M.; Mandić, M.R.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Božić Nedeljković, B.D. Extracts Characterization and In Vitro Evaluation of Potential Immunomodulatory Activities of the Moss Hypnum cupressiforme Hedw. Molecules 2020, 25, 3343. [Google Scholar] [CrossRef]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654–3662. [Google Scholar]
- Klavina, L.; Springe, G.; Steinberga, I.; Mezaka, A.; Ievinsh, G. Seasonal changes of chemical composition in boreonemoral moss species. Environ. Exp. Biol. 2018, 16, 9–19. [Google Scholar]
- Karunen, P. Seasonal changes in lipids of photosynthetically active and senescent parts of Sphagnum fuscum. Lindbergia 1982, 8, 35–44. [Google Scholar]
- Thakur, S.; Kapila, S. Seasonal changes in antioxidant enzymes, polyphenol oxidase enzyme, flavonoids and phenolic content in three leafy liverworts. Lindbergia 2017, 40, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; De Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Hu, L.; Liu, D.; Huang, J.; Lin, W. Circumdatin D Exerts Neuroprotective Effects by Attenuating LPS-Induced Pro-Inflammatory Responses and Downregulating Acetylcholinesterase Activity In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Waterman, M.J.; Turnbull, J.D.; Ashcroft, M.B.; McKinley, E.; Watling, J.R.; Bramley-Alves, J.; Casanova-Katny, A.; Zuniga, G.; Flexas, J. It is hot in the sun: Antarctic mosses have high temperature optima for photosynthesis despite cold climate. Front. Plant Sci. 2020, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Gorzolka, K.; Bruelheide, H.; Neumann, S. Seasonal variation of secondary metabolites in nine different bryophytes. Ecol. Evol. 2018, 8, 9105–9117. [Google Scholar] [CrossRef]
- Kashyap, R.; Csintalan, Z.; Veres, K.; Péli, E.R. Seasonal variation of antioxidant enzymatic responses in the desiccation-tolerant bryophyte Syntrichia ruralis (Hedw.) Web. & Mohr. Columella J. Agric. Environ. Sci. 2021, 8, 37–50. [Google Scholar]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Bauer, D.; Soliman, K.F. The antioxidant effects of thymoquinone in activated BV-2 murine microglial cells. Neurochem. Res. 2016, 41, 3227–3238. [Google Scholar] [CrossRef] [Green Version]
- Awada, R.; Rondeau, P.; Grès, S.; Saulnier-Blache, J.S.; d’Hellencourt, C.L.; Bourdon, E. Autotaxin protects microglial cells against oxidative stress. Free Radic. Biol. Med. 2012, 52, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Bazzi, A.; Zeineddine, D.; Jomaa, W.; Daher, A.; Awada, R. Repressive effect of Rhus coriaria L. fruit extracts on microglial cells-mediated inflammatory and oxidative stress responses. J. Ethnopharmacol. 2021, 269, 113748. [Google Scholar] [CrossRef] [PubMed]
- Simonian, N.; Coyle, J. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fu, Y.; Zhang, Y.; Liu, F.; Rose, G.M.; He, X.; Yi, X.; Ren, R.; Li, Y.; Zhang, Y. Butein attenuates the cytotoxic effects of LPS-stimulated microglia on the SH-SY5Y neuronal cell line. Eur. J. Pharmacol. 2020, 868, 172858. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Uang, H.-W.; Lin, R.-J.; Chen, J.; Lo, Y.-C. Neuroprotective effects of glyceryl nonivamide against microglia-like cells and 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. J. Pharmacol. Exp. Ther. 2007, 323, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Peñuelas, N.; Torra, A. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commun. 2019, 10, 973. [Google Scholar] [CrossRef] [Green Version]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative diseases: Regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesner, J.; Kříž, Z.; Kuča, K.; Jun, D.; Koča, J. Acetylcholinesterases—The structural similarities and differences. J. Enzym. Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.; Moore, S. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Uriarte-Pueyo, I.; Calvo, M.I. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Auclair, C.; Voisin, E. Nitroblue tetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; Volume 123. [Google Scholar]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Ber. Der Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biovia, D.S. Discovery Studio, Version 21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepsova, V.; Karasova, J.Z.; Korabecny, J.; Dolezal, R.; Zemek, F.; Bennion, B.J.; Kuca, K. Oximes: Inhibitors of human recombinant acetylcholinesterase. A structure-activity relationship (SAR) study. Int. J. Mol. Sci. 2013, 14, 16882–16900. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunić, T.M.; Mandić, M.R.; Oalđe Pavlović, M.M.; Sabovljević, A.D.; Sabovljević, M.S.; Božić Nedeljković, B.Đ.; Božić, B.Đ. The Influence of Seasonality on Secondary Metabolite Profiles and Neuroprotective Activities of Moss Hypnum cupressiforme Extracts: In Vitro and In Silico Study. Plants 2022, 11, 123. https://doi.org/10.3390/plants11010123
Lunić TM, Mandić MR, Oalđe Pavlović MM, Sabovljević AD, Sabovljević MS, Božić Nedeljković BĐ, Božić BĐ. The Influence of Seasonality on Secondary Metabolite Profiles and Neuroprotective Activities of Moss Hypnum cupressiforme Extracts: In Vitro and In Silico Study. Plants. 2022; 11(1):123. https://doi.org/10.3390/plants11010123
Chicago/Turabian StyleLunić, Tanja M., Marija R. Mandić, Mariana M. Oalđe Pavlović, Aneta D. Sabovljević, Marko S. Sabovljević, Biljana Đ. Božić Nedeljković, and Bojan Đ. Božić. 2022. "The Influence of Seasonality on Secondary Metabolite Profiles and Neuroprotective Activities of Moss Hypnum cupressiforme Extracts: In Vitro and In Silico Study" Plants 11, no. 1: 123. https://doi.org/10.3390/plants11010123