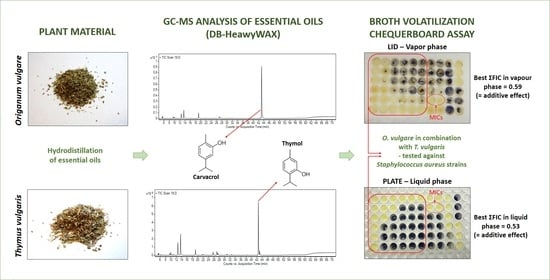
Validation of Qualitative Broth Volatilization Checkerboard Method for Testing of Essential Oils: Dual-Column GC–FID/MS Analysis and In Vitro Combinatory Antimicrobial Effect of Origanum vulgare and Thymus vulgaris against Staphylococcus aureus in Liquid and Vapor Phases
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Analysis
2.2. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Preparation of Essential Oils
4.3. Bacterial Strains and Culture Media
4.4. Antimicrobial Assay
4.5. GC/MS Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, P.N.; Srirama, K.; Dirisala, V.R. An update on clinical burden, diagnostic tools, and therapeutic options of Staphylococcus aureus. Infect. Dis. 2017, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- French, G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 2010, 36, S3–S7. [Google Scholar] [CrossRef]
- Kluytmans, J.A.J.W.; Wertheim, H.F.L. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Bregeon, F.; Mege, J.-L.; Rolain, J.-M.; Blin, O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Prince, A. Staphylococcus aureus infection in the respiratory tract. In Mucosal Immunology of Acute Bacterial Pneumonia; Prince, A., Ed.; Springer: New York, NY, USA, 2013; pp. 239–258. [Google Scholar] [CrossRef]
- MacLeod, D.L.; Velayudhan, J.; Kenney, T.F.; Therrien, J.H.; Sutherland, J.L.; Barker, L.M.; Baker, W.R. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 1529–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curxpharmaceuticals. Available online: http://curxpharma.com/fti.html (accessed on 13 September 2020).
- McCaughey, G.; McKevitt, M.; Elborn, J.S.; Tunney, M.M. Antimicrobial activity of fosfomycin and tobramycin in combination against cystic fibrosis pathogens under aerobic and anaerobic conditions. J. Cyst. Fibros. 2012, 11, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadacek, F. Secondary metabolites as plant traits: Current assessment and future perspectives. Crit. Rev. Plant Sci. 2002, 21, 273–322. [Google Scholar] [CrossRef]
- Leigh-de Rapper, S.; van Vuuren, S.F. Odoriferous therapy: A review identifying essential oils against pathogens of the respiratory tract. Chem. Biodivers. 2020, 17, e2000062. [Google Scholar] [CrossRef]
- Houdkova, M.; Kokoska, L. Volatile antimicrobial agents and in vitro methods for evaluating their activity in the vapour phase: A review. Planta Med. 2020, 86, 822–857. [Google Scholar] [CrossRef]
- Netopilova, M.; Houdkova, M.; Rondevaldova, J.; Kmet, V.; Kokoska, L. Evaluation of in vitro growth-inhibitory effect of carvacrol and thymol combination against Staphylococcus aureus in liquid and vapour phase using new broth volatilization chequerboard method. Fitoterapia 2018, 129, 185–190. [Google Scholar] [CrossRef]
- Netopilova, M.; Houdkova, M.; Urbanova, K.; Rondevaldova, J.; van Damme, P.; Kokoska, L. In vitro antimicrobial combinatory effect of Cinnamomum cassia essential oil with 8-hydroxyquinoline against Staphylococcus aureus in liquid and vapour phase. J. Appl. Microbiol. 2020, 129, 906–915. [Google Scholar] [CrossRef]
- Modnicki, D.; Balcerek, M. Estimation of total polyphenols contents in Ocimum basilicum L., Origanum vulgare L. and Thymus vulgaris L. commercial samples. Herba Pol. 2009, 55, 35–42. [Google Scholar]
- Murillo-Amador, B.; Nieto-Garibay, A.; Lopez-Aguilar, R.; Troyo-Dieguez, E.; Rueda-Puente, E.O.; Flores-Hernandez, A.; Ruiz-Espinosa, F.H. Physiological, morphometric characteristics and yield of Origanum vulgare L. and Thymus vulgaris L. exposed to open-field and shade-enclosure. Ind. Crop. Prod. 2013, 49, 659–667. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Erum, S.; Tabassum, S.; Ameen, F. An overview on medicinal importance of Thymus vulgaris. Asian J. Sci. Res. 2013, 3, 974–982. [Google Scholar]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venancio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Santoro, G.F.; das Gracas, M.C.; Guimaraes, L.G.L.; Salgado, A.P.; Menna-Barreto, R.F.; Soares, M.J. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef]
- Kacaniova, M.; Vukovic, N.; Hleba, L.; Bobkova, A.; Pavelkova, A.; Rovna, K.; Arpasova, H. Antimicrobial and antiradicals activity of Origanum vulgare L. and Thymus vulgaris essential oils. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 263–271. [Google Scholar]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart growing wild in Campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.; Wanner, J.; Hiiferl, M.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Chemical composition, olfactory analysis and antibacterial activity of Thymus vulgaris chemotypes geraniol, 4-thujanol/terpinen-4-ol, thymol and linalool cultivated in southern France. Nat. Prod. Commun. 2012, 7, 1095–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Ozkalp, B.; Sevgi, F.; Ozcan, M.; Ozcan, M.M. The antibacterial activity of essential oil of oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. [Google Scholar]
- de Carvalho, R.J.; de Souza, G.T.; Honorio, V.G.; de Sousa, J.P.; da Conceicao, M.L.; Maganani, M.; de Souza, E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015, 52, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial activity of thyme (Thymus vulgaris) and oregano (Origanum vulgare) essential oils against some food-borne microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Honorio, V.G.; Bezerra, J.; Souza, G.T.; Carvalho, R.J.; Gomes-Neto, N.J.; Figueiredo, R.C.; Melo, J.V.; Souza, E.L.; Magnani, M. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Front Microbiol. 2015, 6, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vuuren, S.F.; Suliman, S.; Viljoen, A.M. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef]
- Stojkovic, D.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Ristic, M.; Siljegovic, J.; Sokovic, M. Investigation on antibacterial synergism of Origanum vulgare and Thymus vulgaris essential oils. Arch. Biol. Sci. 2013, 65, 639–643. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Lopez, P.; Sanchez, C.; Batlle, R.; Nerin, C. Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Nebie, R.H.C.; Yameogo, R.T.; Belanger, A.; Sib, F.S. Composition chimique des huiles essentielles d’Ageratum conyzoides du Burkina Faso. Comptes Rendus Chim. 2004, 7, 1019–1022. [Google Scholar] [CrossRef]
- Lopes, D.; Strobl, H.; Kolodziejczyk, P. 14-Methylpentadecano-15-lactone (Muscolide): A new macrocyclic lactone from the oil of Angelica archangelica L. Chem. Biodivers. 2004, 1, 1880–1887. [Google Scholar] [CrossRef]
- Umano, K.; Shibamoto, T. Analysis of headspace volatiles from overheated beef fat. J. Agric. Food Chem. 1987, 35, 14–18. [Google Scholar] [CrossRef]
- Avato, P.; Raffo, F.; Aldouri, N.A.; Vartanian, S.T. Essential oils of Varthemia iphionoides from Jordan. Flavour Fragr. J. 2004, 19, 559–561. [Google Scholar] [CrossRef]
- Lee, S.-J.; Umano, K.; Shibamoto, T.; Lee, K.-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Kaya, A.; Baser, K.H.C.; Demirci, B.; Koca, F. The essential oil of Acinos alpinus (L.) Moench growing in Turkey. Flavour Fragr. J. 1999, 14, 55–59. [Google Scholar] [CrossRef]
- Ngassoum, M.B.; Yonkeu, S.; Jirovetz, L.; Buchbauer, G.; Schmaus, G.; Hammerschmidt, F.-J.H. Chemical composition of essential oils of Lantana camara leaves and flowers from Cameroon and Madagascar. Flavour Fragr. J. 1999, 14, 245–250. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Prada, N.Q.; Martinez, J.R. HRGC/FID/NP and HRGC/MSD study of Colombian Ylang-Ylang (Cananga odorata) oils obtained by different extraction techniques. J. Res. Chromatogr. 1996, 19, 353–358. [Google Scholar] [CrossRef]
- Bassole, I.H.N.; Ouattara, A.S.; Nebie, R.; Ouattara, C.A.T.; Kabore, Z.I.; Traore, S.A. Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 2003, 62, 209–212. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 1, 228–235. [Google Scholar] [CrossRef]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Abe, S.; Yamaguchi, H.; Asakura, M. Comparative study of antimicrobial and cytotoxic effects of selected essential oils by gaseous and solution contacts. Int. J. Aromather. 2003, 13, 33–41. [Google Scholar] [CrossRef]
- Laird, K.; Phillips, C. Vapour phase: A potential future use for essential oils as antimicrobials? Lett. Appl. Microbiol. 2012, 54, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Czerniewicz, P.; Chrzanowski, G. Antimicrobial activity of five essential oils from Lamiaceae against multidrug-resistant Staphylococcus aureus. Nat. Prod. Res. 2019, 33, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Barros, J.C.; da Conceicao, M.L.; Neto, N.J.; da Costa, A.C. Combined application of Origanum vulgare l. essential oil and acetic acid for controlling the growth of Staphylococcus aureus in foods. Braz. J. Microbiol. 2009, 40, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Kloucek, P.; Smid, J.; Frankova, A.; Kokoska, L.; Valterova, I.; Pavela, R. Fast screening method for assessment of antimicrobial activity of essential oils in vapor phase. Food Res. Int. 2012, 47, 161–165. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Cervantes-Rincon, T.; Bach, H.; Lopez-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod. 2009, 131, 90–95. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.-H. Synergistic activities of gaseous oregano and thyme thymol essential oils against Listeria monocytogenes on surfaces of a laboratory medium and radish sprouts. Food Microbiol. 2020, 86, 103357. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoilova, I.; Bail, S.; Buchbauer, G.; Krastanov, A.; Stoyanova, A.; Schmidt, E.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of an essential oil of Origanum vulgare L. from Bosnia. Nat. Prod. Commun. 2008, 3, 1043–1046. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, M.; Glamoclija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, A.; Markovic, T.; Markovic, D.; Giweli, A.; Sokovic, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M. Composition and antioxidant activity of Thymus vulgaris volatiles: Comparison between supercritical fluid extraction and hydrodistillation. J. Sep. Sci. 2010, 33, 2211–2218. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Mitteregger, U.; Franz, C.; Novak, J. Essential oil compounds of Origanum vulgare L. (Lamiaceae) from Corsica. Nat. Prod. Commun. 2008, 3, 1127–1131. [Google Scholar] [CrossRef] [Green Version]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharmaceut. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Anderson, T.; Parnell, K. Comparison of Cold-Pressed Orange Oil Profiles by GC/MS Using Polar and Non-Polar GC Columns; Report number: TN-2060; Phenomex: Torrance, CA, USA, 2015. [Google Scholar] [CrossRef]
- Fan, S.; Chang, J.; Zong, Y.; Hu, G.; Jia, J. GC-MS Analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules 2018, 23, 576. [Google Scholar] [CrossRef] [Green Version]
- Cachet, T.; Brevard, H.; Chaintreau, A.; Demyttenaere, J.; French, L.; Gassenmeier, K.; Joulain, D.; Koenig, T.; Leijs, H.; Liddle, P.; et al. IOFI recommended practice for the use of predicted relative-response factors for the rapid quantification of volatile flavouring compounds by GC-FID. Flavour Fragr. J. 2016, 31, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- Raquena, R.; Vargas, M.; Chiralt, A. Study of the potential synergistic antibacterial activity of essential oil components using the thiazolyl blue tetrazolium bromide (MTT) assay. LWT 2019, 101, 183–190. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Delgado, B.; Fernandez, P.S.; Palop, A.; Periago, P.M. Effect of thymol and cymene on Bacillus cereus vegetative cells evaluated through the use of frequency distributions. Food Microbiol. 2004, 21, 327–334. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Kuttan, R.; Liju, V.B. Safety evaluation of essential oils. In Essential oils in Food Processing: Chemistry, Safety and Applications, 1st ed.; Hashemi, S.M.B., Khaneghah, A.M., de Sant’Ana, A.S., Eds.; John Wiley & Sons: West Sussex, UK, 2017; pp. 339–358. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA): CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/ (accessed on 15 October 2020).
- Vostinaru, O.; Heghes, S.C.; Filip, L. Safety profile of essential oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications, 1st ed.; de Oliveira, M.S., Ed.; IntechOpen: London, UK, 2020; pp. 1–13. [Google Scholar] [CrossRef] [Green Version]
- Heisterberg, M.V.; Menne, T.; Johansen, J.D. Contact allergy to the 26 specific fragrance ingredients to be declared on cosmetic products in accordance with the EU cosmetics directive. Contact Derm. 2011, 65, 266–275. [Google Scholar] [CrossRef] [PubMed]
- ECHA. European Chemicals Agency. Available online: https://echa.europa.eu/ (accessed on 15 October 2020).
- Xie, K.; Tashkin, D.P.; Luo, M.Z.; Zhang, J.Y. Chronic toxicity of inhaled thymol in lungs and respiratory tracts in mouse model. Pharmacol. Res. Perspect. 2019, 7, e00516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPA. RED Facts: Thymol. EPA-738-F-93–011; Office of Prevention, Pesticides, and Toxic Substances, United States Environmental Protection Agency. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/3143fact.pdf (accessed on 15 October 2020).
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- EMA. European Medicines Agency. Available online: https://www.ema.europa.eu/ (accessed on 19 October 2020).
- AOAC International. Official Methods of Analysis, Official Method 925.10; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2012. [Google Scholar]
- European Pharmacopoeia. Published in Accordance with the Convention on the Elaboration of a European Pharmacopoeia, 7th ed.; European Treaty Series No. 50; Council of Europe: Strasbourg, France, 2013. [Google Scholar]
- Rondevaldova, J.; Hummelova, J.; Tauchen, J.; Kokoska, L. In vitro antistaphylococcal synergistic effect of isoflavone metabolite demethyltexasin with amoxicillin and oxacillin. Microb. Drug Resist. 2018, 24, 24–29. [Google Scholar] [CrossRef] [PubMed]
- NIST WebBook Chemie. NIST Standard Reference Database Number 69. 2020. Available online: http://webbook.nist.gov/chemistry/ (accessed on 5 October 2020).

| S. aureus Strains | MICs Alone (μg/mL) | OVEO at Concentration Indicated in MIC Column in Combination with Listed TVEO Concentrations (μg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OVEO | TVEO | O | +TVEO 512 | +TVEO 256 | +TVEO 128 | +TVEO 64 | +TVEO 32 | ||||||
| MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | ||||
| SA ATCC 25923 | 427 | 427 | ND | 16 | 1.24 | 59 | 0.74 | 149 | 0.65 | 242 | 0.72 | 299 | 0.78 |
| SA ATCC 29213 | 683 | 569 | ND | 16 | 0.94 | 158 | 0.70 | 242 | 0.59 | 398 | 0.70 | 484 | 0.79 |
| SA ATCC 33591 | 626 | 569 | ND | 16 | 0.94 | 112 | 0.63 | 270 | 0.67 | 313 | 0.61 | 370 | 0.65 |
| SA ATCC 33592 | 796 | 484 | ND | 16 | 1.09 | 149 | 0.72 | 370 | 0.73 | 512 | 0.78 | 512 | 0.72 |
| SA ATCC 43300 | 512 | 512 | ND | 16 | 1.03 | 92 | 0.68 | 228 | 0.69 | 398 | 0.90 | 455 | 0.95 |
| SA ATCC BAA 976 | 484 | 484 | ND | 16 | 1.10 | 82 | 0.70 | 228 | 0.74 | 341 | 0.84 | 341 | 0.78 |
| SA 1 | 683 | 512 | ND | 16 | 1.02 | 92 | 0.64 | 242 | 0.63 | 341 | 0.63 | 455 | 0.76 |
| SA 2 | 683 | 626 | ND | 16 | 0.86 | 178 | 0.67 | 313 | 0.67 | 427 | 0.76 | 484 | 0.78 |
| SA 3 | 455 | 455 | ND | 16 | 1.20 | 62 | 0.73 | 185 | 0.72 | 270 | 0.76 | 341 | 0.85 |
| SA 4 | 484 | 484 | ND | 16 | 1.10 | 92 | 0.73 | 194 | 0.67 | 348 | 0.85 | 356 | 0.80 |
| SA 5 | 427 | 427 | ND | 16 | 1.24 | 44 | 0.70 | 149 | 0.64 | 270 | 0.77 | 370 | 0.96 |
| SA 6 | 740 | 796 | ND | 43 | 0.75 | 341 | 0.81 | 427 | 0.76 | 512 | 0.79 | 512 | 0.74 |
| S. aureus Strains | MICs Alone (μg/mL) | OVEO at Concentration Indicated in MIC Column in Combination with Listed TVEO Concentrations (μg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OVEO | TVEO | O | +TVEO 512 | +TVEO 256 | +TVEO 128 | +TVEO 64 | +TVEO 32 | ||||||
| MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | ||||
| SA ATCC 25923 | 512 | 512 | 0.6 | 16 | 1.03 | 156 | 0.81 | 313 | 0.86 | 512 | 1.13 | 512 | 1.06 |
| SA ATCC 29213 | 740 | 569 | 0.4 | 16 | 0.94 | 270 | 0.81 | 341 | 0.69 | 455 | 0.73 | 512 | 0.76 |
| SA ATCC 33591 | 910 | 683 | 555 | 16 | 0.77 | 370 | 0.78 | 484 | 0.73 | 512 | 0.66 | 512 | 0.61 |
| SA ATCC 33592 | 740 | 512 | 164 | 16 | 1.02 | 121 | 0.67 | 341 | 0.72 | 512 | 0.88 | 512 | 0.81 |
| SA ATCC 43300 | 626 | 512 | 36 | 16 | 1.03 | 185 | 0.79 | 313 | 0.75 | 512 | 0.96 | 512 | 0.90 |
| SA ATCC BAA 976 | 569 | 512 | 64 | 16 | 1.03 | 142 | 0.75 | 284 | 0.75 | 427 | 0.89 | 512 | 0.98 |
| SA 1 | 512 | 512 | 6 | 16 | 1.03 | 128 | 0.75 | 284 | 0.81 | 512 | 1.13 | 512 | 1.06 |
| SA 2 | 1024 | 967 | 149 | 16 | 0.55 | 427 | 0.68 | 512 | 0.63 | 512 | 0.57 | 512 | 0.53 |
| SA 3 | 512 | 512 | 455 | 16 | 1.03 | 116 | 0.73 | 256 | 0.75 | 398 | 0.90 | 512 | 1.06 |
| SA 4 | 512 | 512 | 427 | 16 | 1.03 | 121 | 0.74 | 256 | 0.75 | 484 | 1.07 | 512 | 1.06 |
| SA 5 | 512 | 512 | 1 | 16 | 1.03 | 107 | 0.71 | 313 | 0.86 | 512 | 1.13 | 512 | 1.06 |
| SA 6 | 967 | 796 | 1 | 44 | 0.75 | 356 | 0.72 | 512 | 0.71 | 512 | 0.62 | 512 | 0.58 |
| 1 RI | Component | 2 C | 3 RF | 4 Column | 5 Identification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-5MS | DB-H.WAX | HP-5MS | DB-H.WAX | ||||||||
| Obs. | Lit. | (%) | c | (%) | c | ||||||
| 1 | 922 a | 924 | α-Thujene | Monoterpene hydrocarbon (MH) | 0.765 | 1.17 | 0.19 | 0.77 | 0.13 | GC/MS, RI | GC/MS |
| 2 | 929 a | 932 | α-Pinene | MH | 0.765 | 0.67 | 0.11 | 0.42 | 0.07 | GC/MS, RI, Std | GC/MS |
| 3 | 945 a | 946 | Camphene | MH | 0.765 | 0.18 | 0.03 | 0.12 | 0.02 | GC/MS, RI, Std | GC/MS |
| 4 | 973 a | 974 | β-Pinene | MH | 0.765 | 0.16 | 0.03 | 0.10 | 0.02 | GC/MS, RI, Std | GC/MS |
| 5 | 991 a | 988 | β-Myrcene | MH | 0.765 | 1.87 | 0.31 | 1.23 | 0.21 | GC/MS, RI | GC/MS |
| 6 | 1005 a | 1002 | α-Phellandrene | MH | 0.765 | 0.14 | 0.02 | 0.08 | 0.01 | GC/MS, RI | GC/MS |
| 7 | 1009 a | 1008 | 3-Carene | MH | 0.765 | 0.08 | 0.01 | 0.06 | 0.01 | GC/MS, RI, Std | GC/MS |
| 8 | 1017 a | 1014 | α-Terpinene | MH | 0.765 | 0.85 | 0.14 | 0.63 | 0.11 | GC/MS, RI, Std | GC/MS |
| 9 | 1028 a | 1025 | p-Cymene | MH | 0.698 | 8.25 | 1.24 | 5.63 | 0.88 | GC/MS, RI, Std | GC/MS |
| 10 | 1061 a | 1054 | γ-Terpinene | MH | 0.765 | 4.52 | 0.74 | 3.33 | 0.57 | GC/MS, RI, Std | GC/MS |
| 11 | 1078 a | 1068 | trans-Sabinene hydrate | Oxygenated monoterpene (MO) | 0.869 | 0.30 | 0.06 | 0.11 | 0.02 | GC/MS, RI | GC/MS |
| 12 | 1110 a | 1095 | Linalool | MO | 0.869 | 0.11 | 0.02 | - | - | GC/MS, RI, Std | - |
| 13 | 1185 a | 1165 | Borneol | MO | 0.869 | 0.06 | 0.01 | 0.58 | 0.11 | GC/MS, RI, Std | GC/MS |
| 14 | 1190 a | 1174 | Terpinen-4-ol | MO | 0.869 | 0.64 | 0.12 | 0.36 | 0.07 | GC/MS, RI | GC/MS |
| 15 | 1302 a | 1289 | Thymol | MO | 0.808 | 0.26 | 0.04 | 0.47 | 0.08 | GC/MS, RI, Std | GC/MS |
| 16 | 1314 a | 1298 | Carvacrol | MO | 0.808 | 77.92 | 13.52 | 82.60 | 15.01 | GC/MS, RI, Std | GC/MS |
| 17 | 1430 a | 1418 | β-Caryophyllene | Sesquiterpene hydrocarbon (SH) | 0.751 | 1.89 | 0.30 | 1.53 | 0.26 | GC/MS, RI, Std | GC/MS |
| 18 | 1466 a | 1452 | Humulene | SH | 0.751 | 0.26 | 0.04 | 0.18 | 0.03 | GC/MS, RI | GC/MS |
| 19 | 1517 a | 1505 | β-Bisabolene | SH | 0.751 | 0.45 | 0.07 | 0.35 | 0.06 | GC/MS, RI | GC/MS |
| 20 | 1181 b | 1185 c | D-Limonene | MH | 0.765 | - | - | 0.15 | 0.03 | - | GC/MS, RI |
| 21 | 1190 b | 1195 d | β-Phellandrene | MH | 0.765 | - | - | 0.15 | 0.03 | - | GC/MS, RI |
| 22 | 1438 b | 1445 e | 1-Octen-3-ol | Others (O) | 0.748 | - | - | 0.22 | 0.04 | - | GC/MS, RI |
| 23 | 1450 b | 1450 f | cis-Sabinene hydrate | MO | 0.869 | - | - | 0.27 | 0.05 | - | GC/MS, RI |
| 24 | 1579 b | 1583 g | Carvacrol methyl ether | O | 0.798 | - | - | 0.36 | 0.06 | - | GC/MS, RI |
| 25 | 1848 b | 1868 h | Carvacrol acetate | O | 0.901 | - | - | 0.06 | 0.01 | - | GC/MS, RI |
| 26 | 1957 b | 1953 d | Caryophyllene oxide | Oxygenated sesquiterpene | 0.830 | - | - | 0.14 | 0.03 | - | GC/MS, RI |
| Chemical classes | |||||||||||
| Monoterpene hydrocarbons | 17.89 | 12.67 | |||||||||
| Oxygenated monoterpenes | 79.29 | 84.39 | |||||||||
| Sesquiterpene hydrocarbons | 2.60 | 2.06 | |||||||||
| Oxygenated sesquiterpenes | - | 0.14 | |||||||||
| Others | - | 0.64 | |||||||||
| Total identified (%) | 99.78 | 99.90 | |||||||||
| 1 RI | Component | 2 C | 3 RF | 4 Column | 5 Identification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-5MS | DB-H.WAX | HP-5MS | DB-H.WAX | ||||||||
| Obs. | Lit. | (%) | c | (%) | c | ||||||
| 1 | 922 a | 924 | α-Thujene | Monoterpene hydrocarbon (MH) | 0.765 | 0.93 | 0.14 | 0.55 | 0.11 | GC/MS, RI | GC/MS |
| 2 | 929 a | 932 | α-Pinene | MH | 0.765 | 1.01 | 0.15 | 0.67 | 0.13 | GC/MS, RI, Std | GC/MS |
| 3 | 944 a | 946 | Camphene | MH | 0.765 | 0.50 | 0.07 | 0.36 | 0.07 | GC/MS, RI, Std | GC/MS |
| 4 | 973 a | 974 | β-Pinene | MH | 0.765 | 0.24 | 0.04 | 0.18 | 0.04 | GC/MS, RI, Std | GC/MS |
| 5 | 991 a | 988 | β-Myrcene | MH | 0.765 | 2.71 | 0.40 | 1.35 | 0.26 | GC/MS, RI | GC/MS |
| 6 | 1005 a | 1002 | α-Phellandrene | MH | 0.765 | 0.16 | 0.02 | 0.12 | 0.02 | GC/MS, RI | GC/MS |
| 7 | 1008 a | 1008 | 3-Carene | MH | 0.765 | 0.08 | 0.01 | 0.08 | 0.02 | GC/MS, RI, Std | GC/MS |
| 8 | 1017 a | 1014 | α-Terpinene | MH | 0.765 | 1.96 | 0.29 | 1.51 | 0.29 | GC/MS, RI, Std | GC/MS |
| 9 | 1029 a | 1025 | p-Cymene | MH | 0.698 | 24.08 | 3.22 | 18.00 | 3.22 | GC/MS, RI, Std | GC/MS |
| 10 | 1061 a | 1054 | γ-Terpinene | MH | 0.765 | 13.37 | 1.96 | 10.61 | 2.08 | GC/MS, RI, Std | GC/MS |
| 11 | 1078 a | 1068 | trans-Sabinene hydrate | Oxygenated monoterpene (MO) | 0.869 | 0.59 | 0.10 | 0.24 | 0.05 | GC/MS, RI | GC/MS |
| 12 | 1090 a | 1086 | Isoterpinolene | MH | 0.765 | 0.18 | 0.03 | - | - | GC/MS, RI | - |
| 13 | 1113 a | 1095 | Linalool | MO | 0.869 | 2.84 | 0.47 | 2.87 | 0.64 | GC/MS, RI, Std | GC/MS |
| 14 | 1149 a | 1141 | Camphor | MO | 0.887 | 0.28 | 0.05 | 0.30 | 0.07 | GC/MS, RI, Std | GC/MS |
| 15 | 1184 a | 1165 | Borneol | MO | 0.869 | 0.47 | 0.08 | 1.14 | 0.25 | GC/MS, RI, Std | GC/MS |
| 16 | 1190 a | 1174 | Terpinen-4-ol | MO | 0.869 | 0.91 | 0.15 | - | - | GC/MS, RI | - |
| 17 | 1244 a | 1232 | Thymol methyl ether | Other (O) | 0.798 | 0.77 | 0.12 | 2.06 | 0.42 | GC/MS, RI | GC/MS |
| 18 | 1254 a | 1244 | Carvacrol methyl ether | O | 0.798 | 0.64 | 0.10 | 1.47 | 0.30 | GC/MS, RI | GC/MS |
| 19 | 1289 a | 1285 | Bornyl acetate | O | 0.957 | 0.17 | 0.03 | 0.13 | 0.03 | GC/MS, RI, Std | GC/MS |
| 20 | 1302 a | 1289 | Thymol | MO | 0.808 | 42.34 | 6.55 | 48.46 | 10.04 | GC/MS, RI, Std | GC/MS |
| 21 | 1430 a | 1417 | β-Caryophyllene | Sesquiterpene hydrocarbon (SH) | 0.751 | 3.55 | 0.51 | 2.06 | 0.40 | GC/MS, RI, Std | GC/MS |
| 22 | 1466 a | 1452 | Humulene | SH | 0.751 | 0.10 | 0.02 | 0.10 | 0.02 | GC/MS, RI | GC/MS |
| 23 | 1478 a | 1475 | Geranyl propionate | O | 0.935 | 0.06 | 0.01 | 0.10 | 0.02 | GC/MS, RI | GC/MS |
| 24 | 1487 a | 1478 | γ-Muurolene | SH | 0.751 | 0.17 | 0.02 | 0.23 | 0.04 | GC/MS, RI | GC/MS |
| 25 | 1517 a | 1505 | β-Bisabolene | SH | 0.751 | 0.10 | 0.01 | 0.07 | 0.01 | GC/MS, RI | GC/MS |
| 26 | 1529 a | 1513 | γ-Cadinene | SH | 0.751 | 0.30 | 0.04 | - | - | GC/MS, RI | - |
| 27 | 1535 a | 1522 | δ-Cadinene | SH | 0.751 | 0.38 | 0.05 | 0.70 | 0.14 | GC/MS, RI | GC/MS |
| 28 | 1602 a | 1582 | Caryophyllene oxide | Oxygenated sesquiterpene | 0.830 | 0.37 | 0.06 | 0.40 | 0.09 | GC/MS, RI, Std | GC/MS |
| 29 | 1181 b | 1185 c | D-Limonene | MH | 0.765 | - | - | 0.31 | 0.06 | - | GC/MS, RI |
| 30 | 1192 b | 1199 c | 1,8-Cineole | MO | 0.869 | - | - | 0.64 | 0.14 | - | GC/MS, RI |
| 31 | 1438 b | 1445 e | 1-Octen-3-ol | O | 0.748 | - | - | 1.10 | 0.21 | - | GC/MS, RI |
| 32 | 1450 b | 1450 f | cis-Sabinene hydrate | MO | 0.869 | - | - | 0.68 | 0.15 | - | GC/MS, RI |
| 33 | 1471 b | 1475 d | α-Copaene | SH | 0.751 | - | - | 0.06 | 0.01 | - | GC/MS, RI |
| 34 | 1496 b | 1531 g | Bourbonene | SH | 0.751 | - | - | 0.07 | 0.01 | - | GC/MS, RI |
| 35 | 1799 b | 1804 h | Calamenene | SH | 0.707 | - | - | 0.07 | 0.01 | - | GC/MS, RI |
| 36 | 1824 b | 1840 i | Thymol acetate | O | 0.901 | - | - | 0.19 | 0.04 | - | GC/MS, RI |
| 37 | 2169 b | 2186 i | Carvacrol | MO | 0.808 | - | - | 2.65 | 0.55 | - | GC/MS, RI |
| Chemical classes | |||||||||||
| Monoterpene hydrocarbons | 45.22 | 33.74 | |||||||||
| Oxygenated monoterpenes | 47.43 | 56.98 | |||||||||
| Sesquiterpene hydrocarbons | 4.60 | 3.36 | |||||||||
| Oxygenated sesquiterpenes | 0.37 | 0.40 | |||||||||
| Others | 1.64 | 5.05 | |||||||||
| Total identified [%] | 99.26 | 99.53 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netopilova, M.; Houdkova, M.; Urbanova, K.; Rondevaldova, J.; Kokoska, L. Validation of Qualitative Broth Volatilization Checkerboard Method for Testing of Essential Oils: Dual-Column GC–FID/MS Analysis and In Vitro Combinatory Antimicrobial Effect of Origanum vulgare and Thymus vulgaris against Staphylococcus aureus in Liquid and Vapor Phases. Plants 2021, 10, 393. https://doi.org/10.3390/plants10020393
Netopilova M, Houdkova M, Urbanova K, Rondevaldova J, Kokoska L. Validation of Qualitative Broth Volatilization Checkerboard Method for Testing of Essential Oils: Dual-Column GC–FID/MS Analysis and In Vitro Combinatory Antimicrobial Effect of Origanum vulgare and Thymus vulgaris against Staphylococcus aureus in Liquid and Vapor Phases. Plants. 2021; 10(2):393. https://doi.org/10.3390/plants10020393
Chicago/Turabian StyleNetopilova, Marie, Marketa Houdkova, Klara Urbanova, Johana Rondevaldova, and Ladislav Kokoska. 2021. "Validation of Qualitative Broth Volatilization Checkerboard Method for Testing of Essential Oils: Dual-Column GC–FID/MS Analysis and In Vitro Combinatory Antimicrobial Effect of Origanum vulgare and Thymus vulgaris against Staphylococcus aureus in Liquid and Vapor Phases" Plants 10, no. 2: 393. https://doi.org/10.3390/plants10020393