Shades of Fine Dark Chocolate Colors: Polyphenol Metabolomics and Molecular Networking to Enlighten the Brown from the Black
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chocolate Samples
2.3. Color Analysis
2.4. Color Classification Based on Visual Perception
2.5. Extraction of Chocolate Samples
2.6. UHPLC−ESI−Q−Orbitrap MS Analyses
2.7. Chemometrics
3. Results and Discussion
3.1. Sample Classification from Visual Evaluation
3.2. Data Processing and Statistical Analysis
3.3. Compound Identification
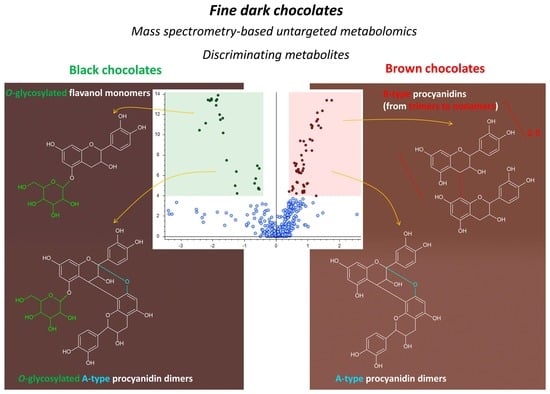
3.3.1. Identification of Discriminating Compounds for Black Chocolates
3.3.2. Identification of Discriminating Compounds for Brown Chocolates
3.4. Considerations on the Origin of Colors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afoakwa, E.O. Chocolate Science and Technology, 1st ed.; Wiley: Chichester, West Sussex, UK, 2016; ISBN 978-1-118-91378-9. [Google Scholar]
- Motamayor, J.C.; Lachenaud, P.; da Silva e Mota, J.W.; Loor, R.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J. Geographic and Genetic Population Differentiation of the Amazonian Chocolate Tree (Theobroma cacao L). PLoS ONE 2008, 3, e3311. [Google Scholar] [CrossRef]
- International Cocoa Organization. Available online: https://www.icco.org/wp-content/uploads/Production_QBCS-XLVIII-No.-3.pdf (accessed on 10 May 2023).
- Giacometti, J.; Jolić, S.M.; Josić, D. Cocoa Processing and Impact on Composition. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 605–612. ISBN 978-0-12-404699-3. [Google Scholar]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From Cocoa to Chocolate: The Impact of Processing on In Vitro Antioxidant Activity and the Effects of Chocolate on Antioxidant Markers In Vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Urbańska, B.; Derewiaka, D.; Lenart, A.; Kowalska, J. Changes in the Composition and Content of Polyphenols in Chocolate Resulting from Pre-Treatment Method of Cocoa Beans and Technological Process. Eur. Food Res. Technol. 2019, 245, 2101–2112. [Google Scholar] [CrossRef]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; Giglio, O.D.; Caggiano, G.; Ciaula, A.D.; Portincasa, P. Chocolate, “Food of the Gods”: History, Science, and Human Health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comprehensive Evaluation of Phenolic Profile in Dark Chocolate and Dark Chocolate Enriched with Sakura Green Tea Leaves or Turmeric Powder. Food Res. Int. 2018, 112, 1–16. [Google Scholar] [CrossRef]
- Zugravu, C.; Otelea, M.R. Dark Chocolate: To Eat or Not to Eat? A Review. J. AOAC Int. 2019, 102, 1388–1396. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Krysiak, W.; Nebesny, E.; Budryn, G. Application of Various Methods for Determination of the Color of Cocoa Beans Roasted under Variable Process Parameters. Eur. Food Res. Technol. 2014, 238, 549–563. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef]
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of Different Conching Processes on Procyanidin Content and Antioxidant Properties of Chocolate. Food Res. Int. 2014, 63, 367–372. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and Quantification of Free and Bound Phenolic Compounds Contained in the High-Molecular Weight Melanoidin Fractions Derived from Two Different Types of Cocoa Beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef]
- Reisdorph, N.; Reisdorph, R.; Quinn, K.; Doenges, K. Metabolomics Mass Spectrometry Data Processing: Applications in Food Analysis. In Comprehensive Foodomics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 339–352. ISBN 978-0-12-816396-2. [Google Scholar]
- Zhang, L.; Cui, D.; Ma, X.; Han, B.; Han, L. Comparative Analysis of Rice Reveals Insights into the Mechanism of Colored Rice via Widely Targeted Metabolomics. Food Chem. 2023, 399, 133926. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Tuenter, E.; Sakavitsi, M.E.; Rivera-Mondragón, A.; Hermans, N.; Foubert, K.; Halabalaki, M.; Pieters, L. Ruby Chocolate: A Study of Its Phytochemical Composition and Quantitative Comparison with Dark, Milk and White Chocolate. Food Chem. 2021, 343, 128446. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Belviso, S.; Ferrocino, I.; Rojo-Poveda, O.; Zeppa, G. Characterization and Classification of Cocoa Bean Shells from Different Regions of Venezuela Using HPLC-PDA-MS/MS and Spectrophotometric Techniques Coupled to Chemometric Analysis. Foods 2021, 10, 1791. [Google Scholar] [CrossRef]
- Kumar, S.; D’Souza, R.N.; Behrends, B.; Corno, M.; Ullrich, M.S.; Kuhnert, N.; Hütt, M.-T. Cocoa Origin Classifiability through LC-MS Data: A Statistical Approach for Large and Long-Term Datasets. Food Res. Int. 2021, 140, 109983. [Google Scholar] [CrossRef]
- Herrera-Rocha, F.; Cala, M.P.; Aguirre Mejía, J.L.; Rodríguez-López, C.M.; Chica, M.J.; Olarte, H.H.; Fernández-Niño, M.; Gonzalez Barrios, A.F. Dissecting Fine-Flavor Cocoa Bean Fermentation through Metabolomics Analysis to Break down the Current Metabolic Paradigm. Sci. Rep. 2021, 11, 21904. [Google Scholar] [CrossRef]
- D’Souza, R.N.; Grimbs, S.; Behrends, B.; Bernaert, H.; Ullrich, M.S.; Kuhnert, N. Origin-Based Polyphenolic Fingerprinting of Theobroma Cacao in Unfermented and Fermented Beans. Food Res. Int. 2017, 99, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Tang, K.; Rao, Z.; Chen, J. Effects of Different Aging Methods on the Phenolic Compounds and Antioxidant Activity of Red Wine. Fermentation 2022, 8, 592. [Google Scholar] [CrossRef]
- Wollgast, J.; Pallaroni, L.; Agazzi, M.-E.; Anklam, E. Analysis of Procyanidins in Chocolate by Reversed-Phase High-Performance Liquid Chromatography with Electrospray Ionisation Mass Spectrometric and Tandem Mass Spectrometric Detection. J. Chromatogr. A 2001, 926, 211–220. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of Procyanidins in Cocoa (Theobroma Cacao) and Chocolate Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef]
- Counet, C.; Collin, S. Effect of the Number of Flavanol Units on the Antioxidant Activity of Procyanidin Fractions Isolated from Chocolate. J. Agric. Food Chem. 2003, 51, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Saputro, A.D.; Van de Walle, D.; Aidoo, R.P.; Mensah, M.A.; Delbaere, C.; De Clercq, N.; Van Durme, J.; Dewettinck, K. Quality Attributes of Dark Chocolates Formulated with Palm Sap-Based Sugar as Nutritious and Natural Alternative Sweetener. Eur. Food Res. Technol. 2017, 243, 177–191. [Google Scholar] [CrossRef]
- Beegum, P.P.S.; Pandiselvam, R.; Ramesh, S.V.; Nooh, A.; Gupta, A.; Varghese, E.; Balasubramanian, D.; Apshara, E.S.; Manikantan, M.R.; Hebbar, K.B. Sensorial, Textural, and Nutritional Attributes of Coconut Sugar and Cocoa Solids Based “Bean-to-bar” Dark Chocolate. J. Texture Stud. 2022, 53, 870–882. [Google Scholar] [CrossRef]
- Briones, V.; Aguilera, J.M.; Brown, C. Effect of Surface Topography on Color and Gloss of Chocolate Samples. J. Food Eng. 2006, 77, 776–783. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Fernández-Prior, Á.; Cancino Chávez, K.; Gómez-Mendoza, J.; Fuente-Carmelino, L.D.L.; Muñoz, A.M. Colour, Fatty Acids, Bioactive Compounds, and Total Antioxidant Capacity in Commercial Cocoa Beans (Theobroma cacao L.). LWT 2021, 147, 111629. [Google Scholar] [CrossRef]
- Barnaba, C.; Nardin, T.; Pierotti, A.; Malacarne, M.; Larcher, R. Targeted and Untargeted Characterisation of Free and Glycosylated Simple Phenols in Cocoa Beans Using High Resolution-Tandem Mass Spectrometry (Q-Orbitrap). J. Chromatogr. A 2017, 1480, 41–49. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- De Taeye, C.; Caullet, G.; Eyamo Evina, V.J.; Collin, S. Procyanidin A2 and Its Degradation Products in Raw, Fermented, and Roasted Cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, P.; Chen, K.; Jia, Q.; Li, Y. Oxidative Conversion of B- to A-Type Procyanidin Trimer: Evidence for Quinone Methide Mechanism. Food Chem. 2014, 154, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Poupard, P.; Sanoner, P.; Baron, A.; Renard, C.M.G.C.; Guyot, S. Characterization of Procyanidin B2 Oxidation Products in an Apple Juice Model Solution and Confirmation of Their Presence in Apple Juice by High-Performance Liquid Chromatography Coupled to Electrospray Ion Trap Mass Spectrometry: LC-ESI-MS/MS of Procyanidin B2 Oxidation Products. J. Mass Spectrom. 2011, 46, 1186–1197. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Merino-Zegarra, C.; Tang, M.; Pertino, M.W.; Simirgiotis, M.J. UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants 2022, 11, 595. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, P.-L.; Chen, X.-L.; Gong, M.-J.; Zhang, L.; Qiu, X.-H.; Zhang, J.; Huang, Z.-H.; Xu, W. The Impact of Citrus-Tea Cofermentation Process on Chemical Composition and Contents of Pu-Erh Tea: An Integrated Metabolomics Study. Front. Nutr. 2021, 8, 737539. [Google Scholar] [CrossRef]
- de Sousa Dias, A.L.; Verbaere, A.; Meudec, E.; Deshaies, S.; Saucier, C.; Cheynier, V.; Sommerer, N. Improved Analysis of Isomeric Polyphenol Dimers Using the 4th Dimension of Trapped Ion Mobility Spectrometry—Mass Spectrometry. Molecules 2022, 27, 4176. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of C -Glycosyl Flavonoids in the Human Diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef]
- Stark, T.; Hofmann, T. Application of a Molecular Sensory Science Approach to Alkalized Cocoa ( Theobroma Cacao): Structure Determination and Sensory Activity of Nonenzymatically C-Glycosylated Flavan-3-Ols. J. Agric. Food Chem. 2006, 54, 9510–9521. [Google Scholar] [CrossRef]
- Fayeulle, N.; Vallverdu-Queralt, A.; Meudec, E.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Characterization of New Flavan-3-Ol Derivatives in Fermented Cocoa Beans. Food Chem. 2018, 259, 207–212. [Google Scholar] [CrossRef]
- Fayeulle, N.; Meudec, E.; Boulet, J.C.; Vallverdu-Queralt, A.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Fast Discrimination of Chocolate Quality Based on Average-Mass-Spectra Fingerprints of Cocoa Polyphenols. J. Agric. Food Chem. 2019, 67, 2723–2731. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; Herrero, M.; Decker, E.A.; López-Giraldo, L.J.; Ibáñez, E. Preparative Separation of Procyanidins from Cocoa Polyphenolic Extract: Comparative Study of Different Fractionation Techniques. Molecules 2020, 25, 2842. [Google Scholar] [CrossRef] [PubMed]
- Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 6 January 2023).
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Misnawi1; Jinap, S.; Jamilah, B.; Nazamid, S. Effects of Incubation and Polyphenol Oxidase Enrichment on Colour, Fermentation Index, Procyanidins and Astringency of Unfermented and Partly Fermented Cocoa Beans. Int. J. Food Sci. Technol. 2003, 38, 285–295. [Google Scholar] [CrossRef]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Structural Determination of Colourless and Yellow Dimers Resulting from (+)-Catechin Coupling Catalysed by Grape Polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar] [CrossRef]
- Verloop, A.J.W.; Gruppen, H.; Vincken, J.-P. Annotation of Different Dehydrocatechin Oligomers by MS/MS and Their Occurrence in Black Tea. J. Agric. Food Chem. 2016, 64, 6011–6023. [Google Scholar] [CrossRef] [PubMed]
- Germann, D.; Stark, T.D.; Hofmann, T. Formation and Characterization of Polyphenol-Derived Red Chromophores. Enhancing the Color of Processed Cocoa Powders: Part 1. J. Agric. Food Chem. 2019, 67, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Germann, D.; Stark, T.D.; Hofmann, T. Formation and Characterization of Polyphenol-Derived Red Chromophores. Enhancing the Color of Processed Cocoa Powders: Part 2. J. Agric. Food Chem. 2019, 67, 4643–4651. [Google Scholar] [CrossRef]
- Feng, H.; Li, Y.; Wang, S.; Zhang, L.; Liu, Y.; Xue, F.; Sun, Y.; Wang, Y.; Sun, J. Molecular Analysis of Proanthocyanidins Related to Pigmentation in Brown Cotton Fibre (Gossypium hirsutum L.). J. Exp. Bot. 2014, 65, 5759–5769. [Google Scholar] [CrossRef]





| Color Classification a | Sample Code (Year b) |
|---|---|
| Brown | 184 (2019), 185 (2019), 51 (2020), 186 (2019), 57 (2020), 50 (2020), 15 (2020), 54 (2020) |
| Intermediate | 183 (2019), 17 (2020), 19 (2020), 55 (2020), 20 (2020), 56 (2020), 18 (2020), 14 (2020), 58 (2020), 180 (2019), 13 (2020), 16 (2020), 21 (2020), 53 (2020), 205 (2019), 210 (2019), 52 (2020), 204 (2019), 179 (2019), 209 (2019), 207 (2019) |
| Black | 206 (2019), 217 (2019), 182 (2019), 214 (2019), 215 (2019), 181 (2019), 178 (2019), 216 (2019) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, A.L.d.S.; Fenger, J.-A.; Meudec, E.; Verbaere, A.; Costet, P.; Hue, C.; Coste, F.; Lair, S.; Cheynier, V.; Boulet, J.-C.; et al. Shades of Fine Dark Chocolate Colors: Polyphenol Metabolomics and Molecular Networking to Enlighten the Brown from the Black. Metabolites 2023, 13, 667. https://doi.org/10.3390/metabo13050667
Dias ALdS, Fenger J-A, Meudec E, Verbaere A, Costet P, Hue C, Coste F, Lair S, Cheynier V, Boulet J-C, et al. Shades of Fine Dark Chocolate Colors: Polyphenol Metabolomics and Molecular Networking to Enlighten the Brown from the Black. Metabolites. 2023; 13(5):667. https://doi.org/10.3390/metabo13050667
Chicago/Turabian StyleDias, Aecio Luís de Sousa, Julie-Anne Fenger, Emmanuelle Meudec, Arnaud Verbaere, Pierre Costet, Clotilde Hue, Florent Coste, Sophie Lair, Véronique Cheynier, Jean-Claude Boulet, and et al. 2023. "Shades of Fine Dark Chocolate Colors: Polyphenol Metabolomics and Molecular Networking to Enlighten the Brown from the Black" Metabolites 13, no. 5: 667. https://doi.org/10.3390/metabo13050667