Chitosan Enhances Intestinal Health in Cats by Altering the Composition of Gut Microbiota and Metabolites
Abstract
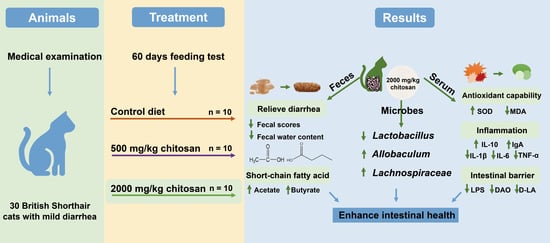
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Animals and Experimental Treatments
2.3. Feeding and Sample Collection
2.4. Serum Parameters Measurement
2.5. Hematological Parameters
2.6. Determination of Fecal SCFAs
2.7. Fecal Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Physical Characteristics
3.2. Hematological Parameters
3.3. Fecal SCFAs
3.4. Serum Anti-Oxidative Condition
3.5. Serum Intestinal Barrier Function Parameters
3.6. Serum Inflammatory Cytokines and Immunoglobulins
3.7. Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Score | Standard |
| 1 | Dry, hard, easy to pick up, difficult to defecate, and forms a ball of dung |
| 2 | Forming, not hard, segmented, and easy to pick up |
| 3 | Wet, no section, and not easy to pick up |
| 4 | Wet, columnar, and difficult to pick up |
| 5 | Wet, heaped, and hard to pick up |
| 6 | Shapeless and heaped/shoal |
| 7 | Watery diarrhea |
References
- Hou, Q.; Huang, J.; Ayansola, H.; Masatoshi, H.; Zhang, B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front. Immunol. 2020, 11, 623691. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.R.; Davis, N.; Hoyles, L. Review article: The human intestinal virome in health and disease. Aliment. Pharmacol. Ther. 2017, 46, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [Green Version]
- Moon, C.D.; Young, W.; Maclean, P.H.; Cookson, A.L.; Bermingham, E.N. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiologyopen 2018, 7, e00677. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, G.; Argentini, C.; Milani, C.; Turroni, F.; Cristina Ossiprandi, M.; van Sinderen, D.; Ventura, M. Catching a glimpse of the bacterial gut community of companion animals: A canine and feline perspective. Microb. Biotechnol. 2020, 13, 1708–1732. [Google Scholar] [CrossRef]
- Rochus, K.; Janssens, G.P.; Hesta, M. Dietary fibre and the importance of the gut microbiota in feline nutrition: A review. Nutr. Res. Rev. 2014, 27, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Ziese, A.-L.; Suchodolski, J.S. Impact of Changes in Gastrointestinal Microbiota in Canine and Feline Digestive Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 155–169. [Google Scholar] [CrossRef]
- Kil, D.Y.; Swanson, K.S. Companion animals symposium: Role of microbes in canine and feline health. J. Anim. Sci. 2011, 89, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Wu, Z.X.; Yin, F.W.; Song, S.; Li, A.; Zhu, B.W.; Yu, L.L. Chitosan and Derivatives: Bioactivities and Application in Foods. Annu. Rev. Food Sci. Technol. 2021, 12, 407–432. [Google Scholar] [CrossRef]
- Tong, A.J.; Hu, R.K.; Wu, L.X.; Lv, X.C.; Li, X.; Zhao, L.N.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Zhao, Z.; Pi, X.; Meng, Y.; Fei, D.; Liu, D.; Wang, X. Effect of chitooligosaccharides on human gut microbiota and antiglycation. Carbohydr. Polym. 2020, 242, 116413. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in in vitro Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2021, 60, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academies Press: Washington, DC, USA, 2006; p. 424.
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan Oligosaccharide Reduces Intestinal Inflammation That Involves Calcium-Sensing Receptor (CaSR) Activation in Lipopolysaccharide (LPS)-Challenged Piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut Microbiota and Diarrhea: An Updated Review. Front. Cell Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Zheng, D.; Liao, H.; Chen, S.; Liu, X.; Mao, C.; Zhang, C.; Meng, M.; Wang, Z.; Wang, Y.; Jiang, Q.; et al. Elevated Levels of Circulating Biomarkers Related to Leaky Gut Syndrome and Bacterial Translocation Are Associated with Graves Disease. Front. Endocrinol. 2021, 12, 796212. [Google Scholar] [CrossRef]
- Ji, M.; Huang, H.; Lan, X. Correlation between Intestinal Microflora in Irritable Bowel Syndrome and Severity. Dis. Markers 2022, 2022, 1031844. [Google Scholar] [CrossRef]
- Vella, A.; Farrugia, G. D-lactic acidosis: Pathologic consequence of saprophytism. Mayo. Clin. Proc. 1998, 73, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A. Chapter 15—Gastrointestinal toxicity biomarkers. In Biomarkers in Toxicology; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 269–277. [Google Scholar]
- Tao, W.; Wang, G.; Pei, X.; Sun, W.; Wang, M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants 2022, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- John, L.J.; Fromm, M.; Schulzke, J.D. Epithelial barriers in intestinal inflammation. Antioxid. Redox Signal. 2011, 15, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Bamias, G.; Cominelli, F. Cytokines and intestinal inflammation. Curr. Opin. Gastroenterol. 2016, 32, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; OGarra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, 20190418. [Google Scholar] [CrossRef] [Green Version]
- Na, K.; Wei, J.; Zhang, L.; Fang, Y.; Li, X.; Lu, S.; Guo, X. Effects of chitosan oligosaccharides (COS) and FMT from COS-dosed mice on intestinal barrier function and cell apoptosis. Carbohydr. Polym. 2022, 297, 120043. [Google Scholar] [CrossRef]
- Rojas, O.L.; Pröbstel, A.K.; Porfilio, E.A.; Wang, A.A.; Charabati, M.; Sun, T.; Lee, D.S.W.; Galicia, G.; Ramaglia, V.; Ward, L.A.; et al. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell 2019, 176, 610–624.E18. [Google Scholar] [CrossRef] [Green Version]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Wang, Y.Z.; Wu, Y.X.; Zhang, M.Y.; Li, F.T.; He, Y.; Wen, L.K.; Yue, H. Effect of stabilization malvids anthocyanins on the gut microbiota in mice with oxidative stress. J. Food Biochem. 2021, 45, 4892–4902. [Google Scholar] [CrossRef]
- Todorova, I.; Simeonova, G.; Kyuchukova, D.; Dinev, D.; Gadjeva, V. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp. Clin. Pathol. 2005, 13, 190–194. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Jia, S.; Zhang, J.; Dai, X.; Sun, Y. Effect of Chitosan Oligosaccharide on Learning and Memory Functions and Serum Antioxidant Status in a Rat Model of Amyloid-beta1-42-Induced Alzheimer’s Disease. Food Sci. 2017, 38, 220–225. [Google Scholar]
- Mei, Q.X.; Hu, J.H.; Huang, Z.H.; Fan, J.J.; Huang, C.L.; Lu, Y.Y.; Wang, X.P.; Zeng, Y. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol. Sin. 2021, 42, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Ferket, P.R.; Hong, Q.H.; Zhou, J.; Cao, G.T.; Zhou, L.; Chen, A.G. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012, 90, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, Y.; Chen, S.; Hu, M.; Wang, Z.; Wu, G.; Ma, X.; Chen, Z.; Zheng, C. Low-Molecular-Weight Chitosan Supplementation Increases the Population of Prevotella in the Cecal Contents of Weanling Pigs. Front. Microbiol. 2017, 8, 2182. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Effect of chito-oligosaccharides over human faecal microbiota during fermentation in batch cultures. Carbohydr. Polym. 2016, 137, 617–624. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- van Muijlwijk, G.H.; van Mierlo, G.; Jansen, P.; Vermeulen, M.; Bleumink-Pluym, N.M.C.; Palm, N.W.; van Putten, J.P.M.; de Zoete, M.R. Identification of Allobaculum mucolyticum as a novel human intestinal mucin degrader. Gut. Microbes 2021, 13, 1966278. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Takeuchi, T.; Miyauchi, E.; Kanaya, T.; Kato, T.; Nakanishi, Y.; Watanabe, T.; Kitami, T.; Taida, T.; Sasaki, T.; Negishi, H.; et al. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature 2021, 595, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Greetham, H.L.; Gibson, G.R.; Giffard, C.; Hippe, H.; Merkhoffer, B.; Steiner, U.; Falsen, E.; Collins, M.D. Allobaculum stercoricanis gen. nov., sp. nov., isolated from canine feces. Anaerobe 2004, 10, 301–307. [Google Scholar] [CrossRef] [PubMed]




| Ingredients (As-Fed Basis) | % |
|---|---|
| Chicken meal | 54.6 |
| Fish meal | 3.21 |
| Rice | 5.35 |
| Poultry fat | 9.73 |
| Fish fat | 2.15 |
| Potato starch | 18.2 |
| Potatoes | 4.85 |
| Taurine | 0.24 |
| Chicken liver powder | 0.60 |
| Salt | 0.49 |
| Vitamin premix 1 | 0.36 |
| Mineral premix 2 | 0.24 |
| Total | 100 |
| Analyzed nutrient levels (on a DM basis) | |
| Dry matter, % | 90.4 |
| Total energy, MJ/kg | 23.4 |
| Crude protein, % | 41.3 |
| Ether extract, % | 22.2 |
| Ash, % | 8.67 |
| Items | CON | L-CS | H-CS | SEM | p-Value |
|---|---|---|---|---|---|
| BW, kg | |||||
| d 0 | 8.340 | 8.310 | 8.326 | 0.097 | 0.993 |
| d 30 | 8.451 | 8.763 | 8.656 | 0.118 | 0.563 |
| d 60 | 8.557 | 8.862 | 8.811 | 0.096 | 0.391 |
| FCW, % | |||||
| d 0 | 78.12 | 78.51 | 77.51 | 0.750 | 0.878 |
| d 30 | 76.87 | 73.33 | 74.37 | 0.642 | 0.064 |
| d 60 | 75.81 a | 70.04 b | 71.42 b | 0.599 | <0.01 |
| Fecal scores | |||||
| d 0 | 5.300 | 5.300 | 5.300 | 0.098 | 1.000 |
| d 30 | 5.100 | 4.300 | 4.600 | 0.146 | 0.074 |
| d 60 | 4.700 a | 3.100 b | 3.700 b | 0.198 | 0.001 |
| ADFI, g/d | 90.62 b | 94.24 ab | 99.04 a | 1.427 | 0.048 |
| ADWI, mL/d | 137.2 | 148.7 | 152.0 | 2.924 | 0.092 |
| Items | CON | L-CS | H-CS | SEM | p-Value |
|---|---|---|---|---|---|
| d 0 | |||||
| WBC, 109/L | 9.810 | 9.210 | 10.25 | 0.359 | 0.510 |
| Lymphocyte, 109/L | 3.600 | 3.900 | 3.780 | 0.277 | 0.912 |
| Monocyte, 109/L | 0.500 | 0.480 | 0.410 | 0.034 | 0.550 |
| Granulocyte, 109/L | 6.070 | 6.470 | 6.820 | 0.446 | 0.801 |
| Lymphocyte, % | 30.88 | 28.18 | 27.07 | 1.696 | 0.657 |
| Monocyte, % | 5.080 | 4.590 | 4.870 | 0.280 | 0.786 |
| Granulocyte, % | 51.97 | 49.63 | 49.23 | 2.282 | 0.877 |
| RBC, 1012/L | 7.691 | 7.275 | 6.493 | 0.298 | 0.258 |
| Hemoglobin, g/L | 121.1 | 118.3 | 120.6 | 3.085 | 0.930 |
| Hematocrit, % | 39.41 | 37.97 | 37.99 | 0.968 | 0.796 |
| MCV, fL | 45.40 | 46.05 | 45.05 | 0.578 | 0.786 |
| MCH, pg | 14.31 b | 15.50 a | 15.38 ab | 0.212 | 0.035 |
| MCHC, g/L | 318.7 | 328.5 | 331.3 | 3.466 | 0.307 |
| RDW, % | 16.02 | 15.51 | 15.71 | 0.182 | 0.532 |
| PLT, 109/L | 250.6 | 246.0 | 238.8 | 19.80 | 0.972 |
| MPV, fL | 9.600 | 9.020 | 8.830 | 0.285 | 0.532 |
| PDW | 15.92 | 15.33 | 14.92 | 0.181 | 0.072 |
| Plateletcrit, % | 0.282 | 0.253 | 0.237 | 0.024 | 0.759 |
| Eosinophil, % | 2.860 | 3.710 | 3.970 | 0.358 | 0.431 |
| d 60 | |||||
| WBC, 109/L | 9.560 | 10.37 | 10.79 | 0.480 | 0.584 |
| Lymphocyte, 109/L | 3.700 | 3.790 | 3.100 | 0.298 | 0.606 |
| Monocyte, 109/L | 0.600 | 0.560 | 0.570 | 0.046 | 0.937 |
| Granulocyte, 109/L | 6.320 | 6.720 | 6.580 | 0.440 | 0.936 |
| Lymphocyte, % | 31.70 | 32.48 | 27.57 | 1.498 | 0.368 |
| Monocyte, % | 5.330 | 5.090 | 5.220 | 0.250 | 0.931 |
| Granulocyte, % | 49.48 | 50.85 | 49.08 | 1.817 | 0.922 |
| RBC, 1012/L | 7.334 | 7.369 | 7.134 | 0.244 | 0.919 |
| Hemoglobin, g/L | 121.7 | 120.2 | 119.6 | 3.107 | 0.963 |
| Hematocrit, % | 38.46 | 37.41 | 38.82 | 1.053 | 0.860 |
| Items (mg/kg) | CON | L-CS | H-CS | SEM | p-Value |
|---|---|---|---|---|---|
| Acetate | 3378 b | 4079 a | 4564 a | 143.0 | <0.01 |
| Propionate | 2703 | 2906 | 3179 | 209.6 | 0.665 |
| Butyrate | 1885 b | 2298 ab | 2645 a | 100.4 | <0.01 |
| Iso-butyrate | 217.6 | 226.1 | 254.1 | 19.04 | 0.729 |
| Valerate | 988.4 | 1037 | 1012 | 91.45 | 0.978 |
| Iso-valerate | 467.1 | 510.7 | 497.4 | 39.38 | 0.905 |
| Caproate | 69.81 | 70.34 | 77.97 | 10.72 | 0.945 |
| Total SCFA | 9710 b | 11128 ab | 12229 a | 345.8 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, R.; Zhang, M.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Chitosan Enhances Intestinal Health in Cats by Altering the Composition of Gut Microbiota and Metabolites. Metabolites 2023, 13, 529. https://doi.org/10.3390/metabo13040529
Mo R, Zhang M, Wang H, Liu T, Liu P, Wu Y. Chitosan Enhances Intestinal Health in Cats by Altering the Composition of Gut Microbiota and Metabolites. Metabolites. 2023; 13(4):529. https://doi.org/10.3390/metabo13040529
Chicago/Turabian StyleMo, Ruixia, Mingrui Zhang, Haotian Wang, Tianyi Liu, Pan Liu, and Yi Wu. 2023. "Chitosan Enhances Intestinal Health in Cats by Altering the Composition of Gut Microbiota and Metabolites" Metabolites 13, no. 4: 529. https://doi.org/10.3390/metabo13040529