Metabolomic Characterisation of Discriminatory Metabolites Involved in Halo Blight Disease in Oat Cultivars Caused by Pseudomonas syringae pv. coronafaciens
Abstract
:1. Introduction
2. Results
2.1. Disease Severity and Symptom Development of Halo Blight in Oat Cultivars
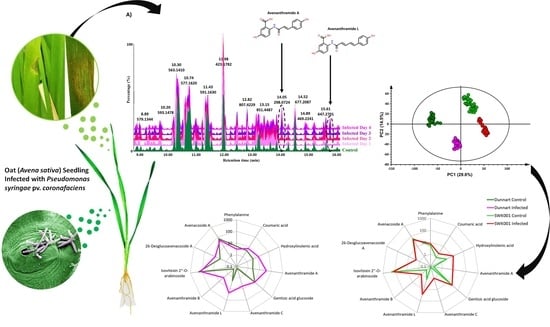
2.2. Liquid Chromatography-Mass Spectrometry-Based Analyses of Oat Response to Ps-c
2.3. Chemometrics for the Analyses of Halo Blight-Induced Metabolic Changes in Oat Cultivars
2.4. Metabolic Profiling of Ps-c Induced Changes in Infected Oat Leaves


3. Discussion
4. Materials and Methods
4.1. Oat Plant Cultivation
4.2. Preparation of Pseudomonas syringae pv. coronafaciens
4.3. Inoculation of Oat Seedlings
4.4. Metabolite Extraction and Sample Preparation
4.5. Sample Analyses Using Ultra-High-Performance Liquid Chromatography (UHPLC)
4.6. Quadrupole Time-of-Flight Mass Spectrometry (q–TOF–MS)
4.7. Data Analysis
4.8. Metabolite Annotation and Semi-Quantitative Comparison
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onaga, G.; Wydra, K. Advances in plant tolerance to abiotic stresses. In Plant Genomics; BoD: Norderstedt, Germany, 2016; pp. 229–272. [Google Scholar]
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant Antimicrobial Agents and Their Effects on Plant and Human Pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019; pp. 1–6. [Google Scholar]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant–Pathogen Interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraire-Velázquez, S.; Balderas-Hernández, V.E. Abiotic stress in plants and metabolic responses. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; InTechOpen: Rijeka, Croatia, 2013; pp. 25–48. [Google Scholar]
- Jorge, T.F.; Mata, A.T.; António, C. Mass Spectrometry as a Quantitative Tool in Plant Metabolomics. Philos. Trans. R. Soc. A 2016, 374, 20150370. [Google Scholar] [CrossRef] [PubMed]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of Secondary Metabolites in Defense Mechanisms of Plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Arruda, R.L.; Paz, A.T.S.; Bara, M.T.F.; Côrtes, M.V.D.C.B.; Filippi, M.C.C.D.; Conceição, E.C.D. An Approach on Phytoalexins: Function, Characterization and Biosynthesis in Plants of the Family Poaceae. Ciênc. Rural 2016, 46, 1206–1216. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Saini, P.; Gani, M.; Saini, P.; Bhat, J.A.; Francies, R.M.; Negi, N.; Chauhan, S.S. Molecular breeding for resistance to economically important diseases of fodder oat. In Disease Resistance in Crop Plants; Wani, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 199–239. [Google Scholar]
- Belobrajdic, D.P.; Bird, A.R. The Potential Role of Phytochemicals in Wholegrain Cereals for the Prevention of Type-2 Diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Chu, Y. Whole Grain Oats, more than just a Fiber: Role of Unique Phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef]
- Dutta, B.; Gitaitis, R.; Agarwal, G.; Coutinho, T.; Langston, D. Pseudomonas coronafaciens sp. nov., a New Phytobacterial Species Diverse from Pseudomonas syringae. PLoS ONE 2018, 13, e0208271. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C. Halo Blight of Oats. J. Agric. Res. 1920, 19, 72–139. [Google Scholar]
- Wilkie, J.P. Halo Blight of Oats in New Zealand. J. Agric. Res. 1972, 15, 461–468. [Google Scholar]
- Harder, D.E.; Harris, D.C. Halo Blight of Oats in Kenya. East Afric. Agric. For. J. 1973, 38, 241–245. [Google Scholar] [CrossRef]
- Kim, Y.C. First Report of Oat Halo Blight Caused by Pseudomonas coronafaciens in South Korea. Plant Dis. 2020, 104, 1853. [Google Scholar] [CrossRef]
- Persson, P.; Sletten, A. Halo blight of oats in Scandinavia. In Plant Pathogenic Bacteria; De Boer, S.H., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 265–268. [Google Scholar]
- Martinelli, J.A. Major Diseases on Oats in South America. In Proceedings of the Sixth International Oat Conference, Lincoln University, Canterbury, New Zealand, 13–16 November 2000; Volume 6, pp. 277–283. [Google Scholar]
- Lamichhane, J.R.; Varvaro, L.; Parisi, L.; Audergon, J.M.; Morris, C.E. Disease and Frost Damage of Woody Plants Caused by Pseudomonas syringae: Seeing the Forest for the Trees. Adv. Agron. 2014, 26, 235–295. [Google Scholar]
- Jones, J.D.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Pretorius, C.J.; Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Plant Metabolomics for Biomarker Discovery: Key Signatory Metabolic Profiles for the Identification and Discrimination of Oat Cultivars. Metabolites 2021, 11, 165. [Google Scholar] [CrossRef]
- Gorash, A.; Armonienė, R.; Mitchell Fetch, J.; Liatukas, Ž.; Danytė, V. Aspects in Oat Breeding: Nutrition Quality, Nakedness and Disease Resistance, Challenges and Perspectives. Ann. Appl. Biol. 2017, 171, 281–302. [Google Scholar] [CrossRef]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for Plant Improvement: Status and Prospects. Front. Plant Sci. 2017, 8, 1302. [Google Scholar] [CrossRef] [Green Version]
- Tekauz, A. A Numerical Scale to Classify Reactions of Barley to Pyrenophora teres. Can. J. Plant Pathol. 1985, 7, 181–183. [Google Scholar] [CrossRef]
- An, J.H.; Noh, Y.H.; Kim, Y.E.; Lee, H.I.; Cha, J.S. Development of PCR and TaqMan PCR Assays to Detect Pseudomonas coronafaciens, a Causal Agent of Halo Blight of Oats. Plant Pathol. J. 2015, 31, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harveson, R.M.; Schwartz, H.F. Bacterial Diseases of Dry Edible Beans in the Central High Plains. Plant Health Prog. 2007, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Riley, M.; Williamson, M.; Maloy, O. Plant Disease Diagnosis. Plant Health Instr. 2002, 10. [Google Scholar] [CrossRef]
- Tugizimana, F.; Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Metabolomics in Plant Priming Research: The Way Forward? Int. J. Mol. Sci. 2018, 19, 1759. [Google Scholar] [CrossRef] [Green Version]
- Preston, G.M. Profiling the Extended Phenotype of Plant Pathogens: Challenges in Bacterial Molecular Plant Pathology. Mol. Plant Pathol. 2017, 18, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Tugizimana, F.; Piater, L.; Dubery, I. Plant Metabolomics: A New Frontier in Phytochemical Analysis. S. Afr. J. Sci. 2013, 109, 1–11. [Google Scholar] [CrossRef]
- Alonso, A.; Marsal, S.; Juliã, A. Analytical Methods in Untargeted Metabolomics: State of the Art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Zhang, Z.; Murtagh, F.; Van Poucke, S.; Lin, S.; Lan, P. Hierarchical Cluster Analysis in Clinical Research with Heterogeneous Study Population: Highlighting Its Visualization with R. Ann. Transl. Med. 2017, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Handorf, E.A.; Heckman, C.J.; Darlow, S.; Slifker, M.; Ritterband, L. A Hierarchical Clustering Approach to Identify Repeated Enrollments in Web Survey Data. PLoS ONE 2018, 13, e0204394. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Holmes, A.E.; Lundstedt, T. Chemometrics in Metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. PCA as a Practical Indicator of OPLS-DA Model Reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, L. Dot Plots. Am. Stat. 1999, 53, 276–281. [Google Scholar]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. A Conversation on Data Mining Strategies in LC-MS Untargeted Metabolomics: Pre-Processing and Pre-Treatment Steps. Metabolites 2016, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the Variable Importance in Projection (VIP) and of the Selectivity Ratio (SR) Methods for Variable Selection and Interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wishart, D.S. MetPA: A Web-Based Metabolomics Tool for Pathway Analysis and Visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Falcone-Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Pretorius, C.J.; Zeiss, D.R.; Dubery, I.A. Mini-Review/Perspective/Commentary: The Presence of Oxygenated Lipids in Plant Defense in Response to Biotic Stress: A Metabolomics Appraisal. Plant Signal. Behav. 2021, 1989215. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Ishihara, A.; Iwamura, H. Induction of Anthranilate Synthase Activity by Elicitors in Oats. Z. Nat. C 2002, 57, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xuan Trinh, H.; Imai, S.; Ishihara, A.; Zhang, L.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Analysis of the Involvement of Hydroxyanthranilate Hydroxycinnamoyltransferase and Caffeoyl-CoA 3-O-Methyltransferase in Phytoalexin Biosynthesis in Oat. Mol. Plant Microbe Interact. 2004, 17, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chen, Y.; Meesapyodsuk, D.; Qiu, X. The Biosynthetic Pathway of Major Avenanthramides in Oat. Metabolites 2019, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Mareya, C.R.; Tugizimana, F.; Piater, L.A.; Madala, N.E.; Steenkamp, P.A.; Dubery, I.A. Untargeted Metabolomics Reveal Defensome-Related Metabolic Reprogramming in Sorghum bicolor against Infection by Burkholderia andropogonis. Metabolites 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Tugizimana, F.; Djami-Tchatchou, A.T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic Analysis of Defense-Related Reprogramming in Sorghum bsicolor in Response to Colletotrichum sublineolum Infection Reveals a Functional Metabolic Web of Phenylpropanoid and Flavonoid Pathways. Front. Plant Sci. 2019, 9, 1840. [Google Scholar] [CrossRef] [Green Version]
- Zeiss, D.R.; Mhlongo, M.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Metabolomic Profiling of the Host Response of Tomato (Solanum lycopersicum) Following Infection by Ralstonia solanacearum. Int. J. Mol. Sci. 2019, 20, 3945. [Google Scholar] [CrossRef] [Green Version]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Comparative Metabolic Phenotyping of Tomato (Solanum lycopersicum) for the Identification of Metabolic Signatures in Cultivars Differing in Resistance to Ralstonia solanacearum. Int. J. Mol. Sci. 2018, 19, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhlongo, M.; Piater, L.; Steenkamp, P.; Labuschagne, N.; Dubery, I. Metabolomic Evaluation of Tissue-Specific Defense Responses in Tomato Plants Modulated by PGPR-Priming against Phytophthora capsici Infection. Plants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate Signaling and Manipulation by Pathogens and Insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef]
- Djande, C.Y.H.; Madala, N.E.; Dubery, I.A. Mass Spectrometric Approaches to Study the Metabolism of Jasmonates: Biotransformation of Exogenously Supplemented Methyl Jasmonate by Cell Suspension Cultures of Moringa Oleifera. In Jasmonate in Plant Biology; Humana: New York, NY, USA, 2019; Volume 2085, pp. 211–226. [Google Scholar] [CrossRef]
- Woldemariam, M.; Galis, I.; Baldwin, I.T. Jasmonoyl-l-Isoleucine Hydrolase 1 (JIH1) Contributes to a Termination of Jasmonate Signaling in N. attenuata. Plant Signal. Behav. 2014, 9, e28973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedini, A.; Mercy, L.; Schneider, C.; Franken, P.; Lucic-Mercy, E. Unraveling the Initial Plant Hormone Signaling, Metabolic Mechanisms and Plant Defense Triggering the Endomycorrhizal Symbiosis Behavior. Front. Plant Sci. 2018, 9, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasternack, C.; Strnad, M. Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef] [Green Version]
- Pietryczuk, A.; Biziewska, I.; Imierska, M.; Czerpak, R. Influence of Traumatic Acid on Growth and Metabolism of Chlorella vulgaris under Conditions of Salt Stress. Plant Growth Regul. 2013, 73, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska-Trypuć, A.; Pankiewicz, W.; Czerpak, R. Traumatic Acid Reduces Oxidative Stress and Enhances Collagen Biosynthesis in Cultured Human Skin Fibroblasts. Lipids 2016, 51, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Chamarthi, S.K.; Kumar, K.; Gunnaiah, R.; Kushalappa, A.C.; Dion, Y.; Choo, T.M. Identification of Fusarium Head Blight Resistance Related Metabolites Specific to Doubled-Haploid Lines in Barley. Eur. J. Plant Pathol. 2013, 138, 67–78. [Google Scholar] [CrossRef]
- Yan, Y.; Borrego, E.; Kolomiets, M.V. Jasmonate biosynthesis, perception and function in plant development and stress responses. In Lipid Metabolism; Baez, R.V., Ed.; InTechOpen: Rijeka, Croatia, 2013; pp. 393–442. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, V.; Mohd, A.S.; Ashraf, T. Avenanthramides of Oats: Medicinal Importance and Future Perspectives. Pharmacogn. Rev. 2018, 12, 66. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Steenkamp, P.A.; Dubery, I.A. Phenylpropanoid Defences in Nicotiana Tabacum Cells: Overlapping Metabolomes Indicate Common Aspects to Priming Responses Induced by Lipopolysaccharides, Chitosan and Flagellin. PLoS ONE 2016, 11, e0151350. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wise, M. Avenanthramide Biosynthesis in Oat Cultivars Treated with Systemic Acquired Resistance Elicitors. Cereal Res. Commun. 2013, 41, 255–265. [Google Scholar] [CrossRef]
- Okazaki, Y.; Isobe, T.; Iwata, Y.; Matsukawa, T.; Matsuda, F.; Miyagawa, H.; Ishihara, A.; Nishioka, T.; Iwamura, H. Metabolism of Avenanthramide Phytoalexins in Oats. Plant J. 2004, 39, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, B.; Mangiardi, L.; Cappiello, F.; Romeo, I.; Loffredo, M.R.; Iazzetti, A.; Calcaterra, A.; Goggiamani, A.; Ghirga, F.; Mangoni, M.L.; et al. Naturally-Occurring Alkaloids of Plant Origin as Potential Antimicrobials against Antibiotic-Resistant Infections. Molecules 2020, 25, 3619. [Google Scholar] [CrossRef]
- Chrzanowska, M.; Rozwadowska, M.D. Asymmetric Synthesis of Isoquinoline Alkaloids. Chem. Rev. 2004, 104, 3341–3370. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Zhao, X.; Xia, Y.; Sun, X.; Xie, W.; Ye, Y.; Lu, X.; Xu, G. Deep Annotation of Hydroxycinnamic Acid Amides in Plants Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry and its in silico DataBase. Anal. Chem. 2018, 90, 14321–14330. [Google Scholar] [CrossRef] [Green Version]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites. Trends Plant Sci. 2020, 26, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecio, Ł.; Wawrzyniak-Szołkowska, A.; Oleszek, W.; Stochmal, A. Rapid Analysis of Avenacosides in Grain and Husks of Oats by UPLC–TQ–MS. Food Chem. 2013, 141, 2300–2304. [Google Scholar] [CrossRef]
- Morrissey, J.P.; Wubben, J.P.; Osbourn, A.E. Stagonospora Avenae Secretes Multiple Enzymes that Hydrolyze Oat Leaf Saponins. Mol. Plant-Microbe Interact. 2000, 13, 1041–1052. [Google Scholar] [CrossRef]
- Du Fall, L.A.; Solomon, P.S. Role of Cereal Secondary Metabolites Involved in Mediating the Outcome of Plant-Pathogen Interactions. Metabolites 2011, 1, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and Functional Diversity of Saponins, Biosynthetic Intermediates and Semi-Synthetic Derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef] [Green Version]
- Hiruma, K.; Saijo, Y. Plant Inoculation with the Fungal Leaf Pathogen Colletotrichum higginsianum. In Environmental Responses in Plants; Duque, P., Ed.; Humana Press: New York, NY, USA, 2016; Volume 1398, pp. 313–318. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and Considerations for the Use of System Suitability and Quality Control Samples in Mass Spectrometry Assays Applied in Untargeted Clinical Metabolomic Studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Multi-Platform Metabolomic Analyses of Ergosterol-Induced Dynamic Changes in Nicotiana tabacum Cells. PLoS ONE 2014, 9, e87846. [Google Scholar] [CrossRef] [Green Version]
- MetaCyc. Available online: https://metacyc.org/ (accessed on 10 November 2020).
- PlantCyc. Available online: https://plantcyc.org/ (accessed on 10 November 2020).
- MassBank. Available online: https://massbank.eu/MassBank/Search (accessed on 4 September 2020).
- Dictionary of Natural Products. Available online: www.dnp.chemnetbase.com (accessed on 10 November 2020).
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/kegg/ (accessed on 4 September 2020).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Putative Identification | Molecular Formula | m/z | Rt (min) | Metabolite Class | Cultivar/Condition | |||
|---|---|---|---|---|---|---|---|---|
| Dun (I) | Dun (C) | SWK (I) | SWK (C) | |||||
| Coumaric acid | C9H8O3 | 163.038 | 3.41 | Phenolic acid | • | ○ | - | - |
| Phenylalanine | C9H10NO2 | 164.069 | 1.69 | Amino acid | - | - | • | ○ |
| Tryptophan | C11H12N2O2 | 203.081 | 2.66 | Amino acid | - | - | • | ○ |
| Oxododecanoic acid | C12H22O3 | 213.147 | 17.25 | Fatty acid | - | - | ○ | • |
| Traumatic acid | C12H20O4 | 227.126 | 16.93 | Fatty acid | • | ○ | • | ○ |
| Naringenin | C15H12O5 | 271.148 | 8.37 | Flavonoid | ○ | • | - | - |
| Hydroxylinolenic acid | C18H30O3 | 293.211 | 21.64 | Fatty acid | - | - | • | ○ |
| Avenanthramide A ** | C16H13NO5 | 298.069 | 14.04 | Phenolic amide | • | ○ | - | - |
| Jasmonic acid-valine | C17H27NO4 | 308.092 | 18.36 | JA conjugate | - | - | • | ○ |
| Avenanthramide C ** | C16H13NO6 | 314.065 | 13.06 | Phenolic amide | • | ○ | - | - |
| Gentisic acid glucoside | C13H16O9 | 315.069 | 1.71 | Phenolic acid | - | - | • | ○ |
| Jasmonoyl-isoleucine | C18H29NO4 | 322.202 | 20.45 | JA conjugate | - | - | • | ○ |
| Avenanthramide L | C18H15NO5 | 324.085 | 15.77 | Phenolic amide | • | ○ | • | ○ |
| Trihydroxyoctadecadienoic acid | C18H32O5 | 327.214 | 16.68 | Fatty acid | ○ | • | • | ○ |
| Avenanthramide B ** | C17H15NO6 | 328.082 | 14.51 | Phenolic amide | • | ○ | - | - |
| Coumaroylquinic acid | C16H18O8 | 337.090 | 3.42 | Chlorogenic acid | • | ○ | - | - |
| Feruloylserotonin | C20H20N2O4 | 351.127 | 6.74 | Phenolic amide | • | ○ | - | - |
| Rutamarin | C21H24O5 | 355.159 | 1.58 | Coumarin | - | - | • | ○ |
| Sinapaldehyde glucoside | C17H22O9 | 369.119 | 13.63 | Phenolic | ○ | • | - | - |
| Dihydroferulic acid glucuronide | C16H20O10 | 371.096 | 5.54 | Phenolic | - | - | ○ | • |
| Syringin | C17H24O9 | 371.135 | 16.02 | Phenolic | ○ | • | - | - |
| Sinapic acid glucose | C17H22O10 | 385.116 | 4.52 | Phenolic | ○ | • | - | - |
| Auriculoside | C22H26O10 | 393.175 | 12.22 | Flavonoid | - | - | ○ | • |
| Quercetin dimethyl ether methylbutyrate | C22H22O8 | 413.121 | 16.7 | Flavonoid | - | - | • | ○ |
| Sophoraflavanone G | C25H28O6 | 423.186 | 11.83 | Flavonoid | ○ | • | - | - |
| Vitexin | C21H20O10 | 431.095 | 10.98 | Flavonoid | ○ | • | - | - |
| Clerodin | C24H34O7 | 433.234 | 22.84 | Terpenoid | • | ○ | - | - |
| Isovolubilin | C23H24O9 | 443.133 | 16.91 | Flavonoid | - | - | • | ○ |
| Tubulosine | C29H37N3O3 | 474.261 | 21.13 | Alkaloid | • | ○ | - | - |
| 1-Acyl-sn-glycero-3-phosphoglycerol | C22H42O9P | 481.254 | 22.79 | Phospholipid | • | ○ | - | - |
| Isoamoritin | C31H38O6 | 505.255 | 21.51 | Flavonoid | • | ○ | - | - |
| Formononetin glucoside malonate | C25H23O12 | 515.247 | 14.2 | Flavonoid | - | - | • | ○ |
| Dirhamnosyl-linoleic acid | C28H48O11 | 559.311 | 21.9 | Fatty acid | • | ○ | - | - |
| Isovitexin 2″-O-arabinoside | C26H28O14 | 563.139 # | 10.33 | Flavonoid | ○ | • | ○ | • |
| Vitexin 2″-O-rhamnoside | C27H30O14 | 577.154 | 10.75 | Flavonoid | ○ | • | ○ | • |
| Acacetin 7-O-rutinoside | C28H32O14 | 593.149 # | 11.39 | Flavonoid | ○ | • | ○ | • |
| Kaempferol rhamnoside galacturonide | C27H28O16 | 607.132 | 9.32 | Flavonoid | ○ | • | - | - |
| Linarin monoacetate | C30H34O15 | 633.181 | 9.32 | Flavonoid | ○ | • | - | - |
| Prenylkaempferol diglucoside | C32H38O16 | 677.207 | 14.51 | Flavonoid | ○ | • | - | - |
| Tricin ether glucopyranoside | C33H36O16 | 689.193 # | 13.21 | Flavonoid | ○ | • | ○ | • |
| Palmitoleic-linoleic glucoside | C33H36O16 | 723.382 | 21.85 | Fatty acid conjugate | • | ○ | - | - |
| 26-Desglucoavenacoside A | C45H72O18 | 945.481 | 18.45 | Steroidal saponin | • | ○ | - | - |
| Avenacoside A ** | C51H82O23 | 1063.539 # | 16.58 | Steroidal saponin | ○ | • | ○ | • |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretorius, C.J.; Steenkamp, P.A.; Tugizimana, F.; Piater, L.A.; Dubery, I.A. Metabolomic Characterisation of Discriminatory Metabolites Involved in Halo Blight Disease in Oat Cultivars Caused by Pseudomonas syringae pv. coronafaciens. Metabolites 2022, 12, 248. https://doi.org/10.3390/metabo12030248
Pretorius CJ, Steenkamp PA, Tugizimana F, Piater LA, Dubery IA. Metabolomic Characterisation of Discriminatory Metabolites Involved in Halo Blight Disease in Oat Cultivars Caused by Pseudomonas syringae pv. coronafaciens. Metabolites. 2022; 12(3):248. https://doi.org/10.3390/metabo12030248
Chicago/Turabian StylePretorius, Chanel J., Paul A. Steenkamp, Fidele Tugizimana, Lizelle A. Piater, and Ian A. Dubery. 2022. "Metabolomic Characterisation of Discriminatory Metabolites Involved in Halo Blight Disease in Oat Cultivars Caused by Pseudomonas syringae pv. coronafaciens" Metabolites 12, no. 3: 248. https://doi.org/10.3390/metabo12030248