Identification of a Unique Cytotoxic Thieno[2,3-c]Pyrazole Derivative with Potent and Selective Anticancer Effects In Vitro
Abstract
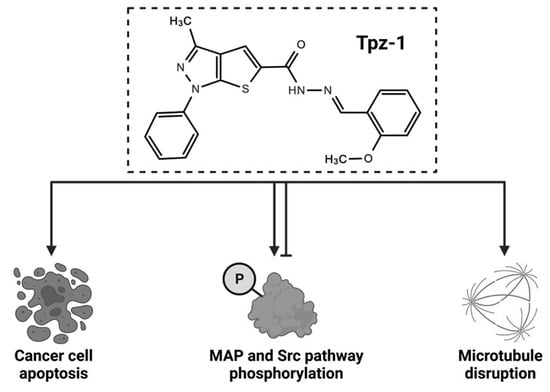
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Preparation of Compounds
2.3. Initial Library Screening and Compound Selection
2.4. Differential Nuclear Staining Assay
2.5. Cytotoxic Concentration 50% and Selective Cytotoxicity Index Values
2.6. AnnexinV-FITC/PI Assay
2.7. Caspase Activation Assays
2.8. Reactive Oxygen Species Assay
2.9. Mitochondrial Depolarization Assay
2.10. Cell Cycle Analysis
2.11. Phospho-Kinase Arrays
2.12. Confocal Microscopy
2.13. Statistical Analysis
3. Results
3.1. Drug Screening Identified a Thienopyrazole Derivative (Tpz-1) with Potent Cytotoxicity against Several Cancer Cell Lines
3.2. Tpz-1 Displays Highly Selective Cytotoxicity against Leukemia and Lymphoma Cell Lines
3.3. Tpz-1 Triggers Movement of Phosphatidylserine to the Outer Plasma Membrane
3.4. Tpz-1 Induces Caspase-Dependent Apoptosis
3.5. Tpz-1 Causes Cell Stress through an Accumulation of Reactive Oxygen Species
3.6. Tpz-1 Treatment Disrupts Mitochondrial Function
3.7. Tpz-1 Cell Death Is Restricted to Cells in the G2/M Phase
3.8. Tpz-1 Interferes with Src and MAP Kinase Function
3.9. Tpz-1 Treatment Impedes Microtubule Organization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gamal, M.I.; Zaraei, S.-O.; Madkour, M.M.; Anbar, H.S. Evaluation of substituted pyrazole-based kinase inhibitors in one decade (2011–2020): Current status and future prospects. Molecules 2022, 27, 330. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.A.; Contreras, L.; Villanueva, P.J.; Borrego, E.A.; Morán-Santibañez, K.; Hess, J.D.; DeJesus, R.; Larragoity, M.; Betancourt, A.P.; Mohl, J.E.; et al. Identification of a potent cytotoxic pyrazole with anti-breast cancer activity that alters multiple pathways. Cells 2022, 11, 254. [Google Scholar] [CrossRef]
- Ashourpour, M.; Mostafavi Hosseini, F.; Amini, M.; Saeedian Moghadam, E.; Kazerouni, F.; Arman, S.Y.; Shahsavari, Z. Pyrazole derivatives induce apoptosis via ROS generation in the triple negative breast cancer cells, MDA-MB-468. Asian Pac. J. Cancer Prev. 2021, 22, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef]
- El-Borai, M.A.; Rizk, H.F.; Ibrahim, S.A.; Fares, A.K.; El-Tahawy, M.M.T.; Beltagy, D.M. Assessment of anti-hemolytic, cytotoxicity, antioxidant activities and molecular docking study based on thienopyrazole scaffold as pharmacophore. J. Mol. Struct. 2021, 1240, 130602. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Cascioferro, S.; Plescia, F.; Cancemi, G.; Daidone, G. Recent advanced in bioactive systems containing pyrazole fused with a five membered heterocycle. Eur. J. Med. Chem. 2015, 97, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Zaki, R.M.; Saber, A.F.; El-Dean, A.M.K.; Radwan, S.M. A concise review on synthesis, reactions and biological importance of thienopyrazoles. ARKIVOC 2020, 2020, 20–60. [Google Scholar] [CrossRef]
- Goto, M.; Murakawa, M.; Kadoshima-Yamaoka, K.; Tanaka, Y.; Inoue, H.; Murafuji, H.; Hayashi, Y.; Miura, K.; Nakatsuka, T.; Nagahira, K.; et al. Phosphodiesterase 7A inhibitor ASB16165 suppresses proliferation and cytokine production of NKT cells. Cell. Immunol. 2009, 258, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Kadoshima-Yamaoka, K.; Murakawa, M.; Yoshioka, R.; Tanaka, Y.; Inoue, H.; Murafuji, H.; Kanki, S.; Hayashi, Y.; Nagahira, K.; et al. Phosphodiesterase 7A inhibitor ASB16165 impairs proliferation of keratinocytes in vitro and in vivo. Eur. J. Pharmacol. 2010, 633, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Brotherton-Pleiss, C.E.; Dillon, M.P.; Ford, A.P.D.W.; Gever, J.R.; Carter, D.S.; Gleason, S.K.; Lin, C.J.; Moore, A.G.; Thompson, A.W.; Villa, M.; et al. Discovery and optimization of RO-85, a novel drug-like, potent, and selective P2X3 receptor antagonist. Bioorg. Med. Chem. Lett. 2010, 20, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Fancelli, D.; Isacchi, A.; Modugno, M.; Moll, J.; Rusconi, L.; Soncini, C.; Lupi, R. Use of a Kinase Inhibitor for the Treatment of Particular Resistant Tumors. U.S. Patent 7,803,829, 28 September 2010. [Google Scholar]
- Fancelli, D.; Moll, J.; Pulici, M.; Quartieri, F.; Bandiera, T. 1H-Thieno[2,3-c]Pyrazole Compounds Useful as Kinase Inhibitors. U.S. Patent 7,943,654, 17 May 2011. [Google Scholar]
- Barberis, C.; Carry, J.-C.; Doerflinger, G.; Barbalat-Damour, D.; Clerc, F.; Minoux, H. Hydrazinocarbonyl-Thieno[2,3-C]Pyrazoles, Process for Preparing Them, Compositions Containing Them and Use Thereof. U.S. Patent 7,595,320, 29 September 2009. [Google Scholar]
- Fayad, W.; Rickardson, L.; Haglund, C.; Olofsson, M.H.; D’Arcy, P.; Larsson, R.; Linder, S.; Fryknäs, M. Identification of agents that induce apoptosis of multicellular tumour spheroids: Enrichment for mitotic inhibitors with hydrophobic properties. Chem. Biol. Drug Des. 2011, 78, 547–557. [Google Scholar] [CrossRef]
- Darwish, W.M.A.; Bayoumi, N.A. Gold Nanorod-Loaded (PLGA-PEG) Nanocapsules as near-infrared controlled release model of anticancer therapeutics. Lasers Med. Sci. 2020, 35, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Hijiya, N.; Ness, K.K.; Ribeiro, R.C.; Hudson, M.M. Acute leukemia as a secondary malignancy in children and adolescents: Current findings and issues. Cancer 2009, 115, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, G.; Mele, L.; Pulsoni, A.; Equitani, F.; Pagano, L. The incidence of secondary leukemias. Haematologica 1999, 84, 937–945. [Google Scholar] [PubMed]
- Bispo, J.A.B.; Pinheiro, P.S.; Kobetz, E.K. Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb. Perspect. Med. 2020, 10, a034819. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Medina, B.E.; Lerma, D.; Hwang, M.; Ross, J.A.; Skouta, R.; Aguilera, R.J.; Kirken, R.A.; Varela-Ramirez, A.; Robles-Escajeda, E. Green barley mitigates cytotoxicity in human lymphocytes undergoing aggressive oxidative stress, via activation of both the Lyn/PI3K/Akt and MAPK/ERK pathways. Sci. Rep. 2019, 9, 6005. [Google Scholar] [CrossRef] [PubMed]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell. Biochem. 2011, 1, 1–14. [Google Scholar]
- Cook, J.D. Linear Interpolation Calculator. Available online: https://www.johndcook.com/interpolator.html (accessed on 26 January 2022).
- Nakamura-Bencomo, S.; Gutierrez, D.A.; Robles-Escajeda, E.; Iglesias-Figueroa, B.; Siqueiros-Cendón, T.S.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Aguilera, R.J.; Rascón-Cruz, Q.; Varela-Ramirez, A. Recombinant human lactoferrin carrying humanized glycosylation exhibits antileukemia selective cytotoxicity, microfilament disruption, cell cycle arrest, and apoptosis activities. Investig. New Drugs 2021, 39, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Robles-Escajeda, E.; Das, U.; Ortega, N.M.; Parra, K.; Francia, G.; Dimmock, J.R.; Varela-Ramirez, A.; Aguilera, R.J. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell. Oncol. 2016, 39, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in mother nature for anti-cancer activity: Anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef] [Green Version]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; DeJesus, R.E.; Contreras, L.; Rodriguez-Palomares, I.A.; Villanueva, P.J.; Balderrama, K.S.; Monterroza, L.; Larragoity, M.; Varela-Ramirez, A.; Aguilera, R.J. A new pyridazinone exhibits potent cytotoxicity on human cancer cells via apoptosis and poly-ubiquitinated protein accumulation. Cell Biol. Toxicol. 2019, 35, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.A.; Sivandzade, F.; Bhalerao, A.; Cucullo, L. Conventional and electronic cigarettes dysregulate the expression of iron transporters and detoxifying enzymes at the brain vascular endothelium: In Vivo evidence of a gender-specific cellular response to chronic cigarette smoke exposure. Neurosci. Lett. 2018, 682, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Reutelingsperger, C.P.; McGahon, A.J.; Rader, J.A.; van Schie, R.C.; LaFace, D.M.; Green, D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, M.; DiPersio, J.F. Small molecule inhibitors in acute myeloid leukemia: From the bench to the clinic. Expert Rev. Hematol. 2014, 7, 439–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- El-Dean, A.M.K.; Zaki, R.M.; Abdulrazzaq, A.Y. A convenient synthesis and biological activity of novel Thieno[2,3-c]Pyrazole compounds as antimicrobial and anti-inflammatory agents. Russ. J. Bioorg. Chem. 2015, 41, 97–104. [Google Scholar] [CrossRef]
- McLean, L.R.; Zhang, Y.; Zaidi, N.; Bi, X.; Wang, R.; Dharanipragada, R.; Jurcak, J.G.; Gillespy, T.A.; Zhao, Z.; Musick, K.Y.; et al. X-Ray Crystallographic Structure-based design of selective thienopyrazole inhibitors for interleukin-2-inducible tyrosine kinase. Bioorg. Med. Chem. Lett. 2012, 22, 3296–3300. [Google Scholar] [CrossRef] [PubMed]
- Akritopoulou-Zanze, I.; Hajduk, P.J. Kinase-targeted libraries: The design and synthesis of novel, potent, and selective kinase inhibitors. Drug Discov. Today 2009, 14, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Akritopoulou-Zanze, I.; Darczak, D.; Sarris, K.; Phelan, K.M.; Huth, J.R.; Song, D.; Johnson, E.F.; Jia, Y.; Djuric, S.W. Scaffold oriented synthesis. part 1: Design, preparation, and biological evaluation of thienopyrazoles as kinase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 96–99. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Black, C.P.; Cowan, K.H. Irradiation-induced G2/M checkpoint response requires ERK1/2 activation. Oncogene 2007, 26, 4689–4698. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Hong, Y.; Miao, L.; Li, C.; Xu, G.; Wei, S.; Wang, B.; Huang, C.; Jiao, B. Wentilactone A as a novel potential antitumor agent induces apoptosis and G2/M arrest of human lung carcinoma cells, and is mediated by HRas-GTP accumulation to excessively activate the Ras/Raf/ERK/P53-P21 pathway. Cell Death Dis. 2013, 4, e952. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in cancer metastasis and translational advances. Biomed. Res. Int. 2013, 2013, 421821. [Google Scholar] [CrossRef]
- Xiao, X.; Li, B.X.; Mitton, B.; Ikeda, A.; Sakamoto, K.M. Targeting CREB for cancer therapy: Friend or foe. Curr. Cancer Drug Targets 2010, 10, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Riebe, C.; Pries, R.; Schroeder, K.N.; Wollenberg, B. Phosphorylation of STAT3 in head and neck cancer requires P38 MAPKinase, whereas phosphorylation of STAT1 occurs via a different signaling pathway. Anticancer. Res. 2011, 31, 3819–3825. [Google Scholar] [PubMed]
- Du, K.; Montminy, M. CREB Is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 1998, 273, 32377–32379. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, B.; Lu, Y.; Guan, Y.; Chen, F. JNK-Dependent Stat3 phosphorylation contributes to Akt activation in response to arsenic exposure. Toxicol. Sci. 2012, 129, 363–371. [Google Scholar] [CrossRef]
- Delghandi, M.P.; Johannessen, M.; Moens, U. The CAMP signalling pathway activates CREB through PKA, P38 and MSK1 in NIH 3T3 cells. Cell. Signal. 2005, 17, 1343–1351. [Google Scholar] [CrossRef]
- Iwamoto, E.; Ueta, N.; Matsui, Y.; Kamijo, K.; Kuga, T.; Saito, Y.; Yamaguchi, N.; Nakayama, Y. ERK plays a role in chromosome alignment and participates in M-phase progression. J. Cell. Biochem. 2016, 117, 1340–1351. [Google Scholar] [CrossRef]
- Verlhac, M.H.; de Pennart, H.; Maro, B.; Cobb, M.H.; Clarke, H.J. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev. Biol. 1993, 158, 330–340. [Google Scholar] [CrossRef]
- Zou, D.; Yang, X.; Tan, Y.; Wang, P.; Zhu, X.; Yang, W.; Jia, X.; Zhang, J.; Wang, K. Regulation of the hematopoietic cell kinase (HCK) by PML/RARα and PU.1 in acute promyelocytic leukemia. Leuk. Res. 2012, 36, 219–223. [Google Scholar] [CrossRef]
- Shivakrupa, R.; Radha, V.; Sudhakar, C.; Swarup, G. Physical and functional interaction between Hck tyrosine kinase and guanine nucleotide exchange factor C3G results in apoptosis, which is independent of C3G catalytic domain. J. Biol. Chem. 2003, 278, 52188–52194. [Google Scholar] [CrossRef] [Green Version]
- Tibaldi, E.; Brunati, A.M.; Massimino, M.L.; Stringaro, A.; Colone, M.; Agostinelli, E.; Arancia, G.; Toninello, A. Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation. J. Cell. Biochem. 2008, 104, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Acín-Pérez, R.; Carrascoso, I.; Baixauli, F.; Roche-Molina, M.; Latorre-Pellicer, A.; Fernández-Silva, P.; Mittelbrunn, M.; Sanchez-Madrid, F.; Pérez-Martos, A.; Lowell, C.A.; et al. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab. 2014, 19, 1020–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Hematological Cancer Cell Lines | ||
|---|---|---|
| Cell Line | Origin | CC50 (µM ± St. Dev) a |
| CCRF-CEM | T-cell leukemia | 0.74 ± 0.18 (24 h) |
| 0.25 ± 0.04 (48 h) | ||
| 0.19 ± 0.01 (72 h) | ||
| HL-60 | Acute myeloid leukemia | 0.95 ± 0.03 (24 h) |
| 0.63 ± 0.03 (48 h) | ||
| 0.27 ± 0.03 (72 h) | ||
| Ramos | Burkitt’s lymphoma | 0.30 ± 0.01 (48 h) |
| Jurkat | T-cell leukemia | 0.41 ± 0.004 (48 h) |
| MOLT-3 | T-cell leukemia | 2.10 ± 0.14 (48 h) |
| NALM6 | B-cell precursor leukemia | 0.24 ± 0.002 (48 h) |
| MM.1S | Multiple myeloma | 0.82 ± 0.08 (48 h) |
| MM.1R | Dexamethasone-resistant multiple myeloma | 0.78 ± 0.02 (48 h) |
| U266 | Multiple myeloma | 0.93 ± 0.13 (48 h) |
| RPMI 8226 | Multiple myeloma | 0.33 ± 0.01 (48 h) |
| Non-Tumorigenic Cell Lines | ||
| Hs27 * | Foreskin fibroblast | 6.11 ± 0.03 (48 h) |
| 5.72 ± 2.29 (72 h) | ||
| Solid Tumor Cell Lines | ||
| MDA-MB-231 | Triple-negative breast adenocarcinoma | 1.94 ± 0.19 (72 h) |
| MDA-MB-468 | Triple-negative breast adenocarcinoma | 0.80 ± 0.07 (72 h) |
| MCF7 | ER+ breast adenocarcinoma | 2.99 ± 0.67 (72 h) |
| COLO 205 | Colorectal adenocarcinoma | 0.93 ± 0.03 (72 h) |
| HT-29 | Colorectal adenocarcinoma | 0.91 ± 0.06 (72 h) |
| HeLa | Cervical adenocarcinoma | 0.68 ± 0.04 (72 h) |
| SH-SY5Y | Neuroblastoma | 0.21 ± 0.01 (72 h) |
| Cell Line | SCI 48 h |
|---|---|
| CCRF-CEM | 24.44 |
| HL-60 | 9.70 |
| Ramos | 20.37 |
| Jurkat | 14.90 |
| MOLT-3 | 2.91 |
| NALM6 | 25.46 |
| MM.1S | 7.45 |
| MM.1R | 7.83 |
| U266 | 6.57 |
| RPMI 8226 | 18.52 |
| Cell Line | SCI 72 h |
| CCRF-CEM | 30.11 |
| HL-60 | 21.19 |
| MDA-MB-231 | 2.95 |
| MDA-MB-468 | 7.15 |
| MCF7 | 1.91 |
| COLO 205 | 6.15 |
| HT-29 | 6.29 |
| HeLa | 8.41 |
| SH-SY5Y | 27.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hess, J.D.; Macias, L.H.; Gutierrez, D.A.; Moran-Santibanez, K.; Contreras, L.; Medina, S.; Villanueva, P.J.; Kirken, R.A.; Varela-Ramirez, A.; Penichet, M.L.; et al. Identification of a Unique Cytotoxic Thieno[2,3-c]Pyrazole Derivative with Potent and Selective Anticancer Effects In Vitro. Biology 2022, 11, 930. https://doi.org/10.3390/biology11060930
Hess JD, Macias LH, Gutierrez DA, Moran-Santibanez K, Contreras L, Medina S, Villanueva PJ, Kirken RA, Varela-Ramirez A, Penichet ML, et al. Identification of a Unique Cytotoxic Thieno[2,3-c]Pyrazole Derivative with Potent and Selective Anticancer Effects In Vitro. Biology. 2022; 11(6):930. https://doi.org/10.3390/biology11060930
Chicago/Turabian StyleHess, Jessica D., Luca H. Macias, Denisse A. Gutierrez, Karla Moran-Santibanez, Lisett Contreras, Stephanie Medina, Paulina J. Villanueva, Robert A. Kirken, Armando Varela-Ramirez, Manuel L. Penichet, and et al. 2022. "Identification of a Unique Cytotoxic Thieno[2,3-c]Pyrazole Derivative with Potent and Selective Anticancer Effects In Vitro" Biology 11, no. 6: 930. https://doi.org/10.3390/biology11060930