The Potential of the Superhydrophobic State to Protect Magnesium Alloy against Corrosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Characterization
3. Results and Discussion
3.1. The Wettability of Superhydrophobic Samples
3.2. Corrosion Resistance
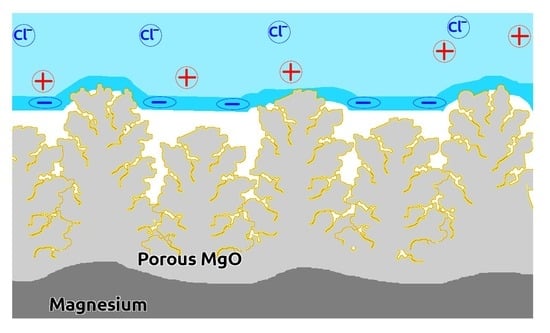
3.3. The Analysis of Protective Mechanisms, Responsible for the Corrosion Resistance of Fabricated Superhydrophobic Coatings on Magnesium Alloy
- The formation of surface texture with good barrier properties during the process of imparting a hierarchical roughness to the surface;
- The minimization of the liquid/solid interaction due to the decrease in the area of real contact between the corrosive liquid and the solid, which is a consequence of the water-repelling properties of coating;
- Chemisorption of the fluorosilane molecules onto the surface-active centers used to decrease the surface energy simultaneously blocks these sites and suppress the adsorption of the corrosive ions onto the surface. Note that such adsorption of corrosive-active ions on the surface is considered as one of the necessary stages of a corrosion process;
- Surface charging and the formation of a double electric layer. Such charging contributes to the depletion of a surface layer of liquid with corrosive ions, thus leading to a decrease in the corrosiveness of a liquid layer adjacent to the surface.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Zhang, D.D.; Peng, F.; Liu, X.Y. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloy. Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Larson, C.; Smith, J.R.; Armstrong, G.J. Current research on surface finishing and coatings for aerospace bodies and structures—A review. Trans. Inst. Met. Finish. 2013, 91, 120–132. [Google Scholar] [CrossRef]
- Barranco, V.; Carmona, N.; Galvan, J.C.; Grobelny, M.; Kwiatkowski, L.; Villegas, M.A. Electrochemical study of tailored sol-gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog. Org. Coat. 2010, 68, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Lkhagvaa, T.; Rehman, Z.U.; Choi, D. Post-anodization methods for improved anticorrosion properties: A review. J. Coat. Technol. Res. 2021, 18, 1–17. [Google Scholar] [CrossRef]
- DeForce, B.S.; Eden, T.J.; Potter, J.K. Cold spray Al-5% Mg coatings for the corrosion protection of magnesium alloys. J. Therm. Spray Technol. 2011, 20, 1352–1358. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, M.; Li, R.H.; Feng, X.Y.; Pang, X.; Rao, J.S.; Cong, D.L.; Yin, C.Q.; Zhang, Y.X. Active corrosion protection of Mg-Al layered double hydroxide for magnesium alloys: A short review. Coatings 2021, 11, 1316. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Watanabe, K.; Suzuki, H. Corrosion resistance of Mg-Al layered double hydroxide container-containing magnesium hydroxide films formed directly on magnesium alloy by chemical-free steam coating. J. Mater. Chem. A 2013, 1, 8968–8977. [Google Scholar] [CrossRef]
- Phuong, N.V.; Gupta, M.; Moon, S. Enhanced corrosion performance of magnesium phosphate conversion coating on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 1087–1095. [Google Scholar] [CrossRef]
- Ishizaki, T.; Shigematsu, I.; Saito, N. Anticorrosive magnesium phosphate coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2009, 203, 2288–2291. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Yin, M.; Pu, J.; Yuan, N.; Ding, J. Superhydrophobic and self-healing Mg-Al layered double hydroxide/silane composite coatings on the Mg alloy surface with a long-term anti-corrosion lifetime. Langmuir 2021, 37, 8129–8138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, J.; Li, B.; Zhao, X.; Zhang, J. Long-term corrosion protection for magnesium alloy by two-layer self-healing superamphiphobic coatings based on shape memory polymers and attapulgite. J. Colloid Interface Sci. 2021, 594, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, W.; Kim, M.J.; Kim, Y.G.; Ko, Y.G. Fabrication of graphene oxide/8-hydroxyquinolin/inorganic coating on the magnesium surface for extraordinary corrosion protection. Prog. Org. Coat. 2019, 137, 105314. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Pashinin, A.S.; Gnedenkov, S.V.; Egorkin, V.S.; Sinebryukhov, S.L. Mg alloy treatment for superhydrophobic anticorrosion coatings formation. Surf. Innov. 2013, 1, 162–172. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.P.; Tian, Y.L. A contrastive investigation on anticorrosive performance of laser-induced super-hydrophobic and oil-infused slippery coatings. Prog. Org. Coat. 2020, 138, 105313. [Google Scholar] [CrossRef]
- Safarpour, M.; Hosseini, S.A.; Ahadani-Targhi, F.; Vasina, P.; Alishahi, M. A transition from petal-state to lotus-state in AZ91 magnesium surface by tailoring the microstructure. Surf. Coat. Technol. 2020, 383, 125239. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Kaminsky, V.V.; Pytskii, I.S.; Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Aleshkin, A.V.; Boinovich, L.B. Antimicrobial activity and degradation of superhydrophobic magnesium substrates in bacterial media. Metals 2021, 11, 1100. [Google Scholar] [CrossRef]
- Joo, J.; Kim, D.; Moon, H.S.; Kim, K.; Lee, J. Durable anti-corrosive oil-impregnated porous surface of magnesium alloy by plasma electrolytic oxidation with hydrothermal treatment. Appl. Surf. Sci. 2020, 509, 145361. [Google Scholar] [CrossRef]
- Saji, V.S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloy. 2021, 9, 748–778. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Emelyanenko, A.M.; Boinovich, L.B. Thermally induced gradient of properties on a superhydrophobic magnesium alloy surface. Metals 2021, 11, 41. [Google Scholar] [CrossRef]
- Venkatesh, R.; Manivannan, S.; Sakthivel, P.; Vijayan, V.; Jidesh, S. The investigation on newly developed of hydrophobic coating on cast AZ91D magnesium alloy under 3.5 wt% NaCl solutions. J. Inorg. Organomet. Polym. Mater. 2021, 29, 1–3. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloy. 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Y.; Wu, L.; Zhang, J.; Pan, F. Effective corrosion and wear protection of slippery liquid-infused porous surface on AZ31 Mg alloy. Surf. Coat. Technol. 2022, 429, 127953. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B. The role of discretization at the video image processing of sessile and pendant drop profiles. Colloids Surf. A 2001, 189, 197–202. [Google Scholar] [CrossRef]
- Chulkova, E.V.; Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Elimination of wetting study flaws in unsaturated vapors based on Laplace fit parameters. Surf. Innov. 2022, 10, 21–24. [Google Scholar] [CrossRef]
- Tan, Q.Y.; Atrens, A.; Mo, N.; Zhang, M.X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef] [Green Version]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Met. 1923, 29, 529–591. [Google Scholar]
- Boinovich, L.B.; Emelyanenko, A.M. The behaviour of fluoro- and hydrocarbon surfactants used for fabrication of superhydrophobic coatings at solid/water interface. Colloids Surf. A 2015, 481, 167–175. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Shiryaev, A.A.; Emelyanenko, K.A.; Dvoretskaya, O.V.; Ganne, A.A. Corrosion behavior of superhydrophobic aluminum alloy in concentrated potassium halide solutions: When the specific anion effect is manifested. Corros. Sci. 2016, 112, 517–527. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Not simply repel water: The diversified nature of corrosion protection by superhydrophobic coatings. Mendeleev Commun. 2017, 27, 254–256. [Google Scholar] [CrossRef]
- Smith, A.L. Applied Infrared Spectroscopy; John Wiley: New York, NY, USA, 1979. [Google Scholar]
- Boinovich, L.B.; Sobolev, V.D.; Maslakov, K.I.; Domantovsky, A.G.; Sergeeva, I.P.; Emelyanenko, A.M. Cation capture and overcharging of a hydrophobized quartz surface in concentrated potassium chloride solutions. Colloids Surf. A 2018, 537, 76–84. [Google Scholar] [CrossRef]








| Element | Cu | Al | Mn | Fe | Si | Ce | Ni | Cu | Zn | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| Content, wt.% | <0.05 | <0.1 | 1.3–2.2 | <0.05 | <0.1 | 0.15–0.35 | <0.007 | <0.05 | <0.3 | Balance |
| Sample | Contact Angle, ° | Roll-Off Angle, ° |
|---|---|---|
| As prepared | 171.5 ± 0.7 | 2.6 ± 0.7 |
| After 30 d of electrochemical tests | 168.2 ± 0.5 | 13.9 ± 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emelyanenko, K.A.; Chulkova, E.V.; Semiletov, A.M.; Domantovsky, A.G.; Palacheva, V.V.; Emelyanenko, A.M.; Boinovich, L.B. The Potential of the Superhydrophobic State to Protect Magnesium Alloy against Corrosion. Coatings 2022, 12, 74. https://doi.org/10.3390/coatings12010074
Emelyanenko KA, Chulkova EV, Semiletov AM, Domantovsky AG, Palacheva VV, Emelyanenko AM, Boinovich LB. The Potential of the Superhydrophobic State to Protect Magnesium Alloy against Corrosion. Coatings. 2022; 12(1):74. https://doi.org/10.3390/coatings12010074
Chicago/Turabian StyleEmelyanenko, Kirill A., Elizaveta V. Chulkova, Alexey M. Semiletov, Alexander G. Domantovsky, Valeria V. Palacheva, Alexandre M. Emelyanenko, and Ludmila B. Boinovich. 2022. "The Potential of the Superhydrophobic State to Protect Magnesium Alloy against Corrosion" Coatings 12, no. 1: 74. https://doi.org/10.3390/coatings12010074