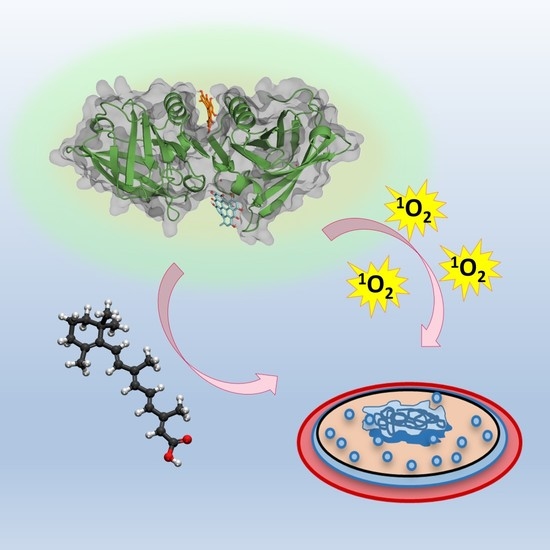
A Double Payload Complex between Hypericin and All-trans Retinoic Acid in the β-Lactoglobulin Protein
Abstract
:1. Introduction
2. Results
2.1. Interaction between Retinoic Acid and Monomeric β-Lactoglobulin
2.2. Interaction between Retinoic Acid and Dimeric β-Lactoglobulin
2.3. Spectroscopic Characterization of the Ternary Complex between β-Lactoglobulin, Retinoic-Acid, and Hypericin
2.4. Antimicrobial Studies
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Spectroscopic Techniques and Computational Chemistry
4.3. Microbial Strains, Culture Conditions, and Photodynamic Inactivation Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Fanelli, M.; Kupperman, E.; Lautenbach, E.; Edelstein, P.H.; Margolis, D.J. Antibiotics, acne, and Staphylococcus aureus colonization. Arch. Dermatol. 2011, 147, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coggeshall, M.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.; Blanc, D.; Cunliffe, W.J. 13-Cis Retinoic Acid and Acne. Lancet 1980, 316, 1048–1049. [Google Scholar] [CrossRef]
- Charakida, A.; Mouser, P.E.; Chu, A.C. Safety and effects of the acne drug, oral isotretinoin. Expert Opin. Drug Saf. 2004, 3, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- de Annunzio, S.R.; de Freitas, L.M.; Blanco, A.L.; da Costa, M.M.; Carmona-Vargas, C.C.; de Oliveira, K.T.; Fontana, C.R. Susceptibility of Enterococcus faecalis and Propionibacterium acnes to antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2018, 178, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Ryu, A.-R.; Jin, S.; Jeon, Y.-M.; Lee, M.-Y. Chlorin e6-Mediated Photodynamic Therapy Suppresses, P. acnes-Induced Inflammatory Response via NFκB and MAPKs Signaling Pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar] [CrossRef] [PubMed]
- Hongcharu, W.; Taylor, C.R.; Aghassi, D.; Suthamjariya, K.; Anderson, R.R.; Chang, Y. Topical ALA-Photodynamic Therapy for the Treatment of Acne Vulgaris. J. Investig. Dermatol. 2000, 115, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.; Lee, J.; Im, B.N.; Park, H.; Na, K. Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance. Biomaterials 2017, 141, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, Y.; Zhao, Z.; Zhang, X.; Liu, P.; Li, C. Efficacy of photodynamic therapy combined with minocycline for treatment of moderate to severe facial acne vulgaris and influence on quality of life. Medicine 2017, 96, e9366. [Google Scholar] [CrossRef]
- Åkerström, B.; Borregaard, N.; Flower, R.; Salier, J.P. Lipocalins; Landes Bioscience: Austin, TX, USA, 2006; ISBN 1-58706-297-6. [Google Scholar]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine β-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Amigo, B.; Delcanale, P.; Rotger, G.; Juárez-Jiménez, J.; Abbruzzetti, S.; Summer, A.; Agut, M.; Luque, F.J.; Nonell, S.; Viappiani, C. The complex of hypericin with β-lactoglobulin has antimicrobial activity with potential applications in dairy industry. J. Dairy Sci. 2015, 98, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Larissa, S.A.; Perussi, J.R. Effectiveness of Hypericin in decreasing the population of Propionibacterium acnes. Photodiagnosis Photodyn. Ther. 2015, 12, 355. [Google Scholar] [CrossRef]
- Futterman, S.; Heller, J. The Enhancement of fluorescence and the decreased susceptibility to enzymatic oxidation of retinol complexed with bovine serum albumin, β-Lactoglobulin, and the retinol-binding protein of human plasma. J. Biol. Chem. 1972, 247, 5168–5172. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Baltimore, MD, USA, 2006. [Google Scholar]
- Liang, L.; Subirade, M. β-Lactoglobulin/Folic Acid Complexes: Formation, Characterization, and Biological Implication. J. Phys. Chem. B 2010, 114, 6707–6712. [Google Scholar] [CrossRef]
- Cheung, H.C. 3. Resonance Energy Transfer. In Topics in Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Plenum Press: New York, NY, USA, 1991; ISBN 978-0-306-47058-5. [Google Scholar]
- McMeekin, T.L.; Groves, M.L.; Hipp, N.J. Refractive Indices of Amino Acids, Proteins, and Related Substances. In Amino Acids and Serum Proteins; Stekol, J.A., Ed.; American Chemical Society: Washington, DC, USA, 1964; pp. 54–66. ISBN 9780841200456. [Google Scholar]
- Tan, M.; Liang, W.; Luo, X.; Gu, Y. Fluorescence spectroscopy study on the interaction between evodiamine and Bovine Serum Albumin. J. Chem. 2013, 2013, 308054. [Google Scholar] [CrossRef]
- Que, Y.-A.; Moreillon, P. 196—Staphylococcus aureus (Including Staphylococcal Toxic Shock Syndrome). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaserett, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 2237–2271. ISBN 978-1-4557-4801-3. [Google Scholar]
- Madigan, M.T.; Martinko, J.M.; Dunlap, P.V.; Clark, D.P. Brock’s Biology of Microorganisms, 8th ed.; Pearson: New York, NY, USA, 1997; ISBN 978-84-7829-136-6. [Google Scholar]
- Lange, D.C.; Kothari, R.; Patel, R.C.; Patel, S.C. Retinol and retinoic acid bind to a surface cleft in bovine beta-lactoglobulin: A method of binding site determination using fluorescence resonance energy transfer. Biophys. Chem. 1998, 74, 45–51. [Google Scholar] [CrossRef]
- Dufour, E.; Marden, M.C.; Haertlé, T. β-lactoglobulin binds retinol and protoporphyrin IX at two different binding sites. FEBS Lett. 1990, 277, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Batt, C.A.; Sawyer, L. Probing the retinol-binding site of bovine β-Lactoglobulin. J. Biol. Chem. 1994, 269, 11102–11107. [Google Scholar] [CrossRef]
- Kontopidis, G.; Holt, C.; Sawyer, L. The Ligand-binding Site of Bovine β-Lactoglobulin: Evidence for a Function? J. Mol. Biol. 2002, 318, 1043–1055. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Lee, H.S.; Jeong, D.; Oh, H.K.; Ra, K.H.; Lee, M.Y. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, S.; Zhang, J. Retinoid combined with photodynamic therapy against hyperkeratotic chromoblastomycosis: A case report and literature review. Mycoses 2021, 64, 18–23. [Google Scholar] [CrossRef]
- Bucher, G.; Bresolí-Obach, R.; Brosa, C.; Flors, C.; Luis, J.G.; Grillo, T.A.; Nonell, S. β-Phenyl quenching of 9-Phenylphenalenone. A novel photocyclisation reaction with biological implications. Phys. Chem. Chem. Phys. 2014, 16, 18813–18820. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Amigo, B.; Hally, C.; Roig-Yanovsky, N.; Delcanale, P.; Abbruzzetti, S.; Agut, M.; Viappiani, C.; Nonell, S. A Double Payload Complex between Hypericin and All-trans Retinoic Acid in the β-Lactoglobulin Protein. Antibiotics 2022, 11, 282. https://doi.org/10.3390/antibiotics11020282
Rodríguez-Amigo B, Hally C, Roig-Yanovsky N, Delcanale P, Abbruzzetti S, Agut M, Viappiani C, Nonell S. A Double Payload Complex between Hypericin and All-trans Retinoic Acid in the β-Lactoglobulin Protein. Antibiotics. 2022; 11(2):282. https://doi.org/10.3390/antibiotics11020282
Chicago/Turabian StyleRodríguez-Amigo, Beatriz, Cormac Hally, Núria Roig-Yanovsky, Pietro Delcanale, Stefania Abbruzzetti, Montserrat Agut, Cristiano Viappiani, and Santi Nonell. 2022. "A Double Payload Complex between Hypericin and All-trans Retinoic Acid in the β-Lactoglobulin Protein" Antibiotics 11, no. 2: 282. https://doi.org/10.3390/antibiotics11020282